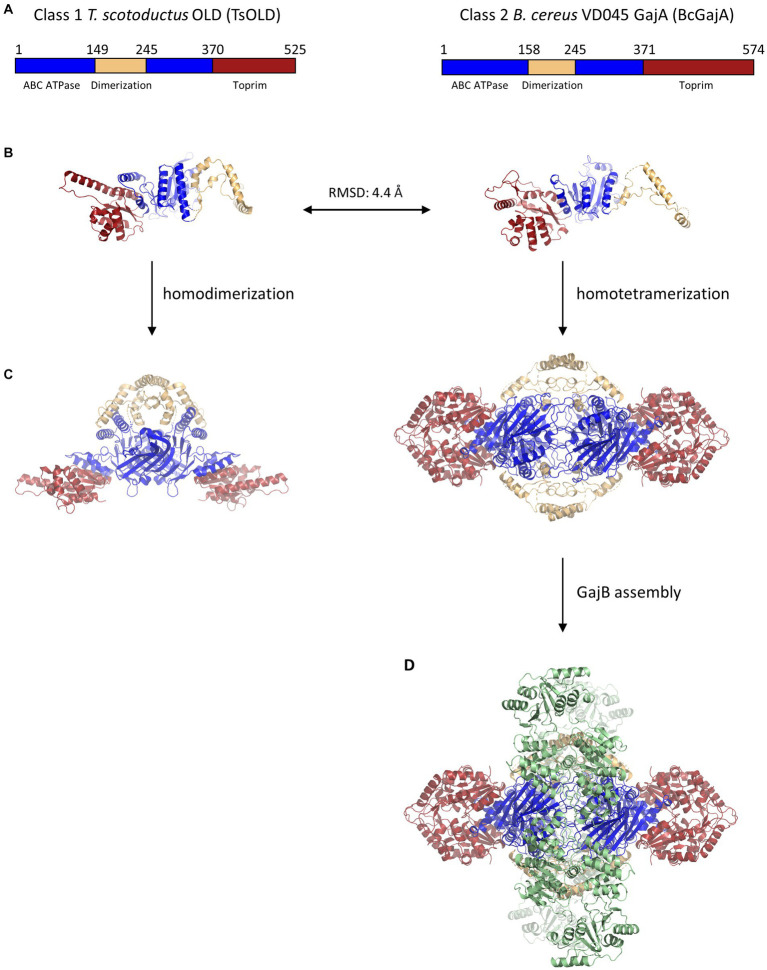
Figure 2.
Structural similarity and multimeric assembly of Class 1 and Class 2 OLD proteins. (A) Schematics showing domain organization within Class 1 T. scotoductus OLD and Class 2 B. cereus GajA OLD proteins. A dimerization domain (tan) is inserted into each ABC ATPase domain (blue). The larger size of BcGajA arises from a helical insert into the Toprim domain (red) that is conserved among Class 2 OLD proteins and makes them larger than Class 1 OLD proteins by about 50 amino acids on average. (B) Structural superposition of TsOLD (PDB: 6P74) and BcGajA (PDB: 8SM3) full-length monomers shows structural similarity (RMSD = 4.4 Å) despite only 22.2% sequence identity. (C) TsOLD dimerizes and BcGajA tetramerizes in crystal structures, but in neither structure are the ATPases poised for ATP hydrolysis. (D) Two separate GajB dimers (green) bind to a GajA homotetramer to assemble an octameric GajAB complex.