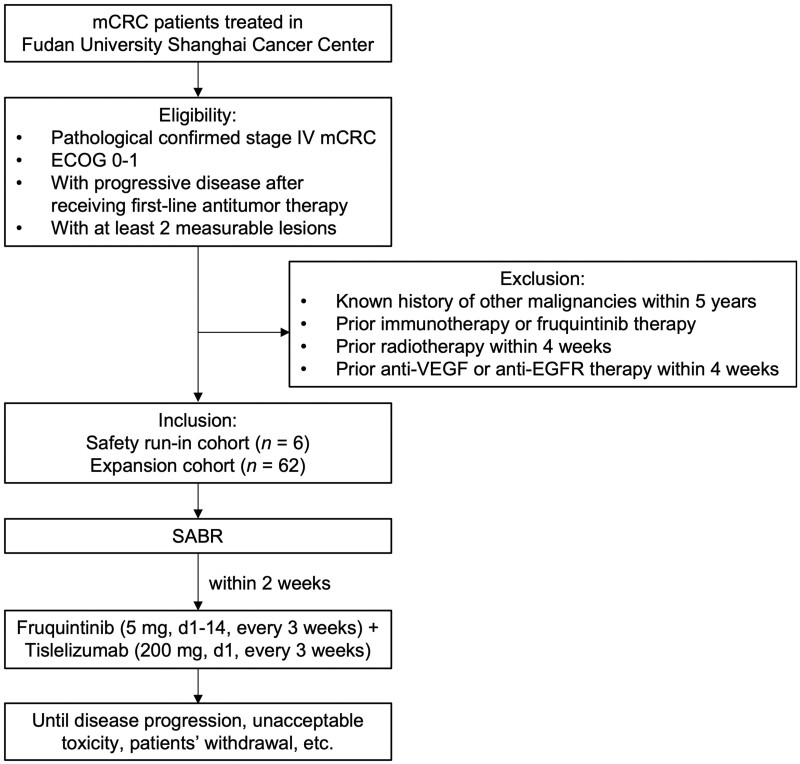
Figure 1.
Flow chart of patient selection. mCRC patients who meet the inclusion and exclusion criteria are recruited in this clinical trial. A total of 68 mCRC patients will be enrolled in the safety run-in phase and the expansion phase. Patients will receive SABR followed by fruquintinib and tislelizumab treatment until disease progression, death, toxicity intolerance, consent withdrawal, etc. ECOG = eastern cooperative oncology group, EGFR = epidermal growth factor receptor, mCRC = metastatic colorectal cancer, SABR = stereotactic ablative radiotherapy, VEGF = vascular endothelial growth factor.