Abstract
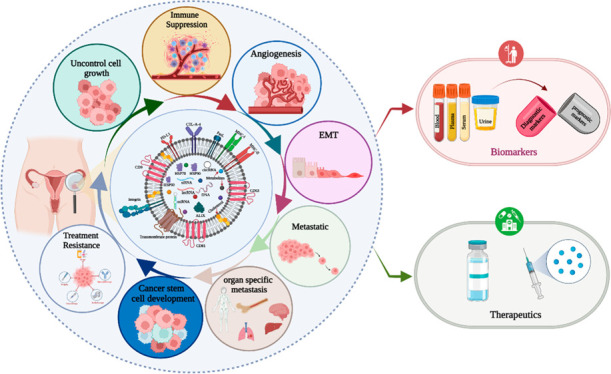
Ovarian cancer (OC) is a common gynecological cancer worldwide. Unfortunately, the lack of early detection methods translates into a substantial cohort of women grappling with the pressing health crisis. The discovery of extracellular vesicles (EVs) (their major subpopulation exosomes, microvesicles, and apoptotic bodies) has provided new insights into the understanding of cancer. Exosomes, a subpopulation of EVs, play a crucial role in cellular communication and reflect the cellular status under both healthy and pathological conditions. Tumor-derived exosomes (TEXs) dynamically influence ovarian cancer progression by regulating uncontrolled cell growth, immune suppression, angiogenesis, metastasis, and the development of drug and therapeutic resistance. In the field of OC diagnostics, TEXs offer potential biomarkers in various body fluids. On the other hand, exosomes have also shown promising abilities to cure ovarian cancer. In this review, we address the interlink between exosomes and ovarian cancer and explore their theragnostic signature. Finally, we highlight future directions of exosome-based ovarian cancer research.
1. Introduction
Ovarian cancer (OC) is a formidable foe that threatens women’s lives.1 OC was the third most common health complication in women worldwide in 2020.2 The complications of ovarian cancer require a smart solution with early detection and a promising therapeutic approach. Extracellular vesicles (EVs) address this requirement. EVs are actively involved in cell-to-cell communication in the tumor microenvironment.3 Biofluid-circulated EVs carry the signature of early stage ovarian cancer markers (diagnostic markers) and prognostic biomarkers.4 EVs Based biomarkers are more effective solution for OC. Exosomes are a subpopulation of EVs that originated from endosomes. They transport several molecular ingredients such as DNA, RNA, proteins, and lipids.5 These components play significant roles in ovarian cancer progression, especially in the early stages of cancer cell development promoted by exosomes.6 Exosomes derived from tumor cells also influence angiogenesis.7 These pesky exosomes also aid in immune system evasion by shutting down the alert function of immune cells like macrophages, dendritic cells, NK cells, B cells, T cells, and myeloid-derived suppressor cells (MDSCs).8 In addition, tumor-derived exosomes (TEXs), derived from ovarian tumor cells, modify the extracellular matrix via fibronectin, resulting in epithelial–mesenchymal transition (EMT).9 This event makes ovarian cancer cells more motile and promotes premetastatic niche formation.10 Ovarian cancer cells can then move across blood vessels and enter the bloodstream.11 The exosome surface intragrain serves as a guide for cellular migration paths and is also linked to the development of ovarian cancer stem cells.12 Advanced ovarian cancer stages can trigger drug and therapeutic resistance via exosomes.13,14 Early detection of ovarian cancer is challenging, but exosome-based investigations offer hope for overcoming this obstacle. Female body fluids, such as blood, plasma, serum, and urine, are excellent sources for exosome-based expression profiling of ovarian cancer biomarkers.15 Exosomes are also potential delivery tools for ovarian cancer therapeutics16,17 and a platform for the development of a strong immune response against ovarian cancer.18 Ongoing clinical trials are exploring the theragnostic potential of exosomes in the treatment and detection of ovarian cancer.18
In this review, we shine a spotlight on the connection between exosomes and ovarian cancer progression, emphasizing their theranostic signatures and the future impact of precision oncology.
2. Unlocking the Mystery Biogenesis of Exosomes
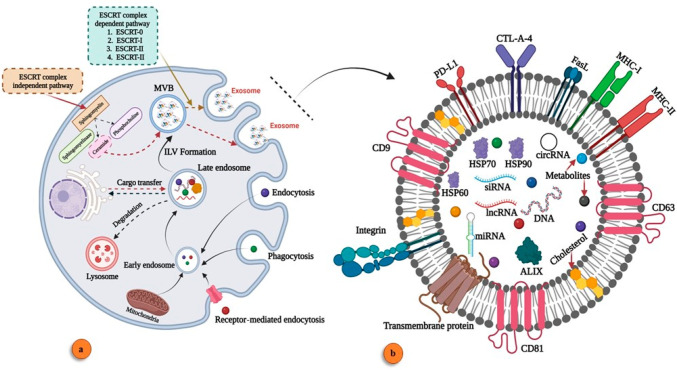
Exosome biogenesis is an intriguing sign of cellular activity. These tiny extracellular vesicles are formed by the fusion of the plasma membrane and multivesicular bodies.19 They contribute to intracellular communication.20 Exosome-based molecular transport (DNA,21 RNA, and proteins) transforms the nature of the recipient cell.22,23 In the cellular system, the maturation and secretion of exosomes go through several stages such as early endosome, late endosome, intraluminal vesicle (ILV), and multivesicular bodies.24,25 The biogenesis of exosomes is classified into two pathways: ESCRT-dependent and ESCRT-independent.26−29 ESCRT-dependent pathways are regulated via the ESCRT complex (ESCRT-0 to ESCRT-III).30 The ESCRT-independent pathway is regulated via a cytoplasmic molecular response, with ceramide playing a vital role in MVB fusion with the plasma membrane and the release of exosomes.31 This pathway has a vital role in cancer cell exosome biogenesis.32 Exosome biogenesis and its molecular signature are illustrated in Figure 1.
Figure 1.
Exosome biogenesis and its component. (a) Exosome biogenesis. (b) Components of exosomes (created with BioRender.com).
3. Unlocking the Puzzle: How TEXs Reprogram the Immune System in Ovarian Cancer
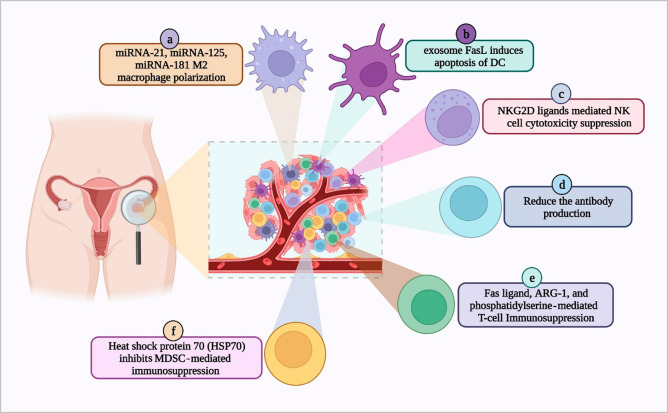
Tumor-derived exosomes (TEXs) carry immunosuppressive or immunostimulatory signaling molecules from the parent tumor cells and promote ovarian origin (Figure 2). TEX-mediated tumor immune microenvironment functional alteration depends on the nature of their payload (miRNAs, or proteins).33 In ovarian cancer (OC), TEXs can alter the activity of macrophages, dendritic cells (DCs), natural killer cells (NK cells), B cells, T cells, and myeloid-derived suppressor cells (MDSCs) for OC development. In the immune system, M2 polarization led to immune suppression for OC development via TEX-derived miRNA-221-3p.34 In OC, TEXs promote premetastasis nish formation.35 The polarization of macrophages to M2 by exosomes plays a significant role in chemoresistance.36 Dendritic cells are involved in the immune system for antigen presentation, but in OC the TEXs surface FasL induces the apoptosis of DCs and suppresses the immune system.37 Natural killer cells are part of the innate immunity, and in OC TEXs surface NKG2D and DNAM-1 receptors reduce the cytotoxic effect of NK cells against cancer.38 B cells play a major role in humoral immunity (antibody production) in the immune system. In OC TEXs reduce the antibody production and suppress humoral immunity.39 T cells are the major player in cell-mediated immunity, and during the OC development event, TEXs FasL and ARG-1 (arginase-1) inhibit the Tell cells development and induce its apoptosis.8,40 Exosomes isolated from the ovarian cancer ascite fluid are responsible for T cell arrest and can cause immunosuppression. Shenoy et al. have shown that the T cell arrest is linked to GD3, a ganglioside expressed on the surface of exosomes.41 Further, ovarian TEXs suppress the T-cell-mediated immune response.42 In OC, blood analysis concludes that a group of exosome-associated molecules present in blood circulation accelerates OC metastasis.43 The higher expression of FasL is one sign of aggressive OC development.44 Research evidence noted that ovarian cancer patients’ liquid biopsies express high levels of FasL.41 TEXs heat shock protein 70 (HSP70) suppresses the myeloid-derived suppressor-cell-mediated anticancer activity.45 Exosomes can also activate myeloid-derived suppressor cells (MDSCs) and regulatory T cells, apart from inducing the differentiation of fibroblasts into cancer-associated phenotypes (cancer-associated fibroblasts CAFs)).41 Thus, the microRNAs, proteins, and lipids transported by the exosomes to the tumor microenvironment can enhance the proliferation of ovarian cancer cells and promote invasion and metastasis. Currently, there are several strategies to tweak the exosome payload or target the exosomes for tumor suppression. Targeting the heat shock protein 70 (HSP70) expressed in the exosomes can inhibit MDSC progression and reprogram the TME for immunosuppression of ovarian cancer.46 Similarly, microRNA-7 enrichment in macrophages can cause tumor suppression via inhibition of the EGFR/AKT/ERK1/2 pathway.47 TEXs-based immune suppression develops an ecosystem for cancer promotion in OC.
Figure 2.
Exosome-mediated immune suppression in ovarian cancer. (a) Macrophage, (b) dendritic cell, (c) natural killer (NK) cells, (d) B-cell, (e) T-cell, and (f) myeloid-derived suppressor cells (MDSCs) (created with BioRender.com).
4. Masterminds of the Battle: How Do TEXs Reshape the Extracellular Matrix in Ovarian Cancer?
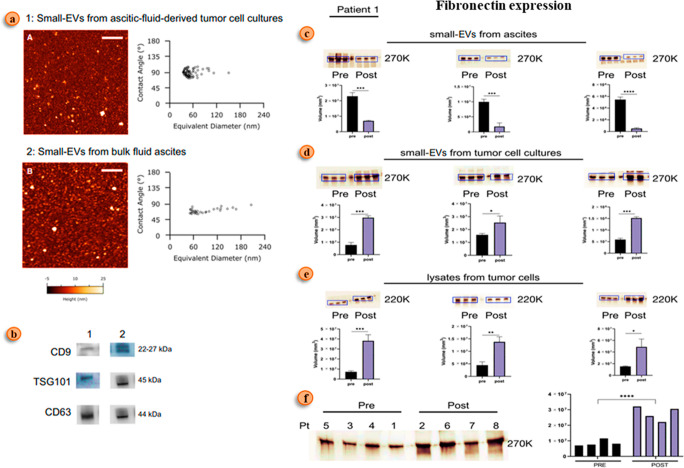
In cancer metastasis, cell development is regulated via a potential event called extracellular matrix (ECM) remodeling.48 TEXs plays a vital role in the ECM remodeling. The underlying mechanism regarding the exact role of these exosomes in the pathogenesis of cancer is still under investigation.49 These tumor-derived exosomes (TEXs) transfer the required genetic information from one cell to another cell (cancer cell to normal cell). They alter the genotypic programming of normal cells, which are present both intracellularly within the cell and extracellularly in the extracellular matrix (ECM). This genetic alteration modifies the cells to actively participate in all cellular events that occur in the progression of cancer, such as angiogenesis, metastasis, etc. Exosomes alter cell genotyping in different cancers.50,51 A study done by José Luis Palacios-Ferrer et al. indicates that exosome oncogenic protein cargos regulate cancer metastasis.52 The ECM is present between the cells, and it comprises cytokines, lymphokines, matrix metalloproteinase (MMPS), and cells like fibroblasts, endothelial cells, and immune cells.53 In cancer, the development phage alters the architecture of the ECM. The collagen structure also affects the treatment outcome in cancer patients. The collagen cross-linking in the ECM influences tumor progression. Lysyl oxidase enzyme, which helps in cross-linking of collagen, increases its rigidity, making it impermeable and promoting tissue fibrosis, thereby enhancing cancer progression.54,55 The collagen alignment in the ECM also gets altered in cancer patients due to the transport of genetic information from tumor cells to normal cells in the ECM by exosomes.51 Cancer cells use this altered direction of collagen molecules to migrate to distant organs, which results in the further spread of tumor tissue. This collagen also provokes cancer cells to dissociate the extracellular matrix by stimulating matrix metalloproteases (MMPs).56 MMP-2 and MMP-9 are expressed in the stromal epithelium of patients with ovarian cancer. They destroy the extracellular matrix as a result of stimulation by cancer cells. Thereafter, collagen molecules will change their direction and help the tumor cells in further metastasis.57,58 In OC, TEX mediates higher fibronectin expression, a key molecule in the ECM, and it also interlinks with OC chemoresistance development (Figure 3).59 TEXs play a significant role in OC progression.
Figure 3.
Fibronectin expression in ovarian cancer patients during chemotherapy. (a) Atomic force microscopic view of ovarian ascite fluid exosomes. (b) Western blot analysis of EV marker: (1) ovarian tumor cells derive EVs and (2) ovarian ascite fluid EVs. (c) Fibronectin expression analysis via Western blot ovarian ascite fluid. (d) Fibronectin expression analysis via Western blot tumor cell lysates. (e) Fibronectin expression analysis via a Western blot patient sample. (f) Fibronectin expression pattern before and after chemotherapy in the clinical sample. Fibronectin is one of the key molecular cargos of EVs that lead to the remodeling of the extracellular matrix (ECM). Adapted with permission from ref (59). Copyright 2021. Molecular Oncology published by John Wiley & Sons Ltd.
5. Unraveling the Enigma: The Role of TEXs in the Progression of Ovarian Cancer
Exosomes, tiny extracellular vesicles, are released by all active cells and tissue types and are surrounded by a lipid bilayer. They facilitate cell-to-cell communication by transporting molecules like DNA, RNA, and proteins.60 Once these biomolecules reach cells, they alter their protein production and gene expression and play a critical role in cancer biology by detecting neoplastic cell progression and metastasis.62 In ovarian cancer, exosomes act as a promising biomarker due to their diverse pathological features.63 Aggressive ovarian-cancer-derived exosomes carry MMP-2, thereby promoting metastasis.12 The molecular cargo of ovarian TEXs, such as CD147, enhances angiogenesis and vascular permeability.64 In the ovarian tumor microenvironment (TME), fibroblast-derived exosomes promote cancer progression.17 Under hypoxic TME conditions, exosomes’ molecular cargos play a role in macrophage polarization.65,66 TEXs mediate higher expression of miRNA-99, which upregulates expression of fibronectin (enhances extracellular matrix remodeling in ovarian cancer) and vimentin (related to the EMT–epithelial–mesenchymal transition).67 In ovarian cancer, immune cell escape is a vital event in preaggregation, with cytokines like IL-6 and STAT3 jointly implicated in immune escape.65 Furthermore, OC-associated TEXs play a key role in cancer progression and metastatic niche formation. For instance, in ovarian cancer ascites, exosomes express ARG1 and FasL, which together downregulate the immune response against cancer.42 The role of exosomes in ovarian cancer progression and metastasis is summarized in Table 1. Exosomes play a critical role in cellular communication and ovarian cancer progression, with their diverse pathological features holding promise as biomarkers for early detection and diagnosis.
Table 1. Exosome Molecular Signature in Ovarian Cancer Progression.
| Cancer development event | Exosome molecule | Function | References |
|---|---|---|---|
| Uncontrolled cell growth | miRNA-29a-3p | Related to develop OC via down-regulation of FOXO3 | (6) |
| Immune response reprogramming | Arginase-1 | Suppress T-cell activity and develop ovarian cancer | (8) |
| Angiogenesis | MALAT1 | Promote angiogenesis-associated gene expression | (7) |
| Metastasis | TGFβ1 | Ovarian tumor microenvironment-related fibroblast-derived exosome cargo associated TGFβ1 promotes EMT via the SMED signaling pathway | (9) |
| Drug and therapeutic resistance | miRNA223 | Macrophage-derived exosome miRNA223 involved developing chemoresistance in ovarian cancer via the PTEN–PI3K/AKT signaling pathway | (68) |
| Cancer stem cell development | CD24 and EpCAM | Both proteins are involved in ovarian cancer stem cell development | (12) |
6. Beyond the Facade: How Do TEXs Drive Epithelial-to-Mesenchymal Transition in Ovarian Cancer?
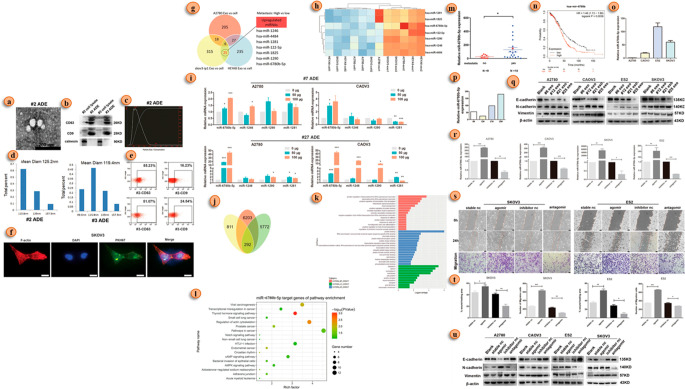
The tumor cells undergo a development wherein the functions related to epithelial cells are subdued and thereby transformed into mesenchymal cells. This change is termed the epithelial–mesenchymal transition (EMT). This is because the properties of mesenchymal cells allow cancer cells to undergo metastasis. A recent study shows that TEXs are associated with the tumor microenvironment alteration that is required for EMT.69,70 The EMT process hinges on a complex web of molecular interactions, involving miRNA, lncRNA, proteins, mRNA, and DNA, all choreographed to facilitate cancer progression,71 which governs the migratory behavior of cancer cells during EMT and contributes to the production of key proteins such as MMP-1, MMP-2, PKM2, and SPARC.72−74 One of the most significant events in EMT is the downregulation of E-cadherin, triggering the upregulation of the fibronectin receptor α5β1-integrin and ultimately leading to metastatic spread.72 Delving deeper, researchers have discovered a direct connection between miRNAs and EMT in ovarian cancer, with various miRNAs indirectly regulating the EMT program.75 As our understanding of the role of miRNA in cancer progression expands,76,77 we uncover new layers of complexity, such as the interactions between malignant cells and healthy cells, the regulation of miRNA through methylation, and the interplay between miRNA and lncRNA.78−80 These lncRNAs, like their molecular counterparts, can either promote or suppress cancerous properties,81 with certain lncRNAs, such as DNM3OS, MEG3, and MIAT, significantly influencing the expression of EMT-related genes.82 Take a captivating visual journey through the signature exosome miRNA in EMT as depicted in Figure 4 and immerse yourself in the fascinating world of cancer cell metamorphosis and its molecular orchestrators.
Figure 4.
Exosome miRNA-led EMT in ovarian cancer. (a) Electron microscopic view of the exosome of a patient with ovarian cancer. (b) Expression of an exosome’s marker (CD63, CD9, and calnexin) in Western blot. (c) Exosomes size analysis using a NTA (nanoparticle tracking assay). (d) Exosome size analysis via DLS (dynamic light scattering). (e) Analysis of exosome marker expression using flow cytometric analysis. (f) Exosome uptake of ovarian cancer cell analysis via immunofluorescence detection (in exosomes, PKH67 was labeled with green, and F-actin was labeled red; nuclei were labeled with DAPI). (g) miRNA analysis in three ovarian cancer cell lines. (h) Seven miRNAs’ higher expression detects in thermograms among 25 miRNAs. (i) qRT-PCR analysis of the seven most expressive miRNAs in three ovarian cancer cell lines. (j) Venn diagram of common higher expression miRNA (miR-6780b-5p). (k) Gene function annotation indicates the target gene of miR-6780b-5p. (l) KEGG analysis of the possible target genes of miR-6780b-5p. (m) qRT-PCR comparative analysis miR-6780b-5p expression. (n) Kaplan–Meier analysis of miR-6780b in pancancer analysis with a K–M plotter. (o) qRT-PCR comparative analysis of miR-6780b-5p expression in four ovarian cancer cell lines. (p) Comparative qRT-PCR analysis of miR-6780b-5p expression in four sets of clinical samples. (q) EMT marker expression in four ovarian cancer cell lines via Western blot (WB). (r) qPCR validation of the effects of the miR-6780b-5p agomir and antagomir in four cell lines. (s) Migration assay analysis after miR-6780b-5p transfection. (t) Migration assay results represented via histograms. (u) WB analysis of EMT marker expression after transfection of miR-6780b-5p. Adapted with permission from ref (83). Copyright 2021. Cell Death and Disease, Springer Nature.
7. Role of TEXs in Ovarian Cancer Organ-Specific Cancer Metastasis
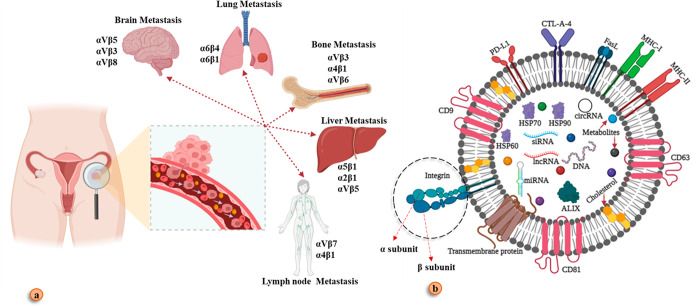
Metastasis is a process by which malignant cells migrate to their secondary site of infection. The cell acquires the ability to migrate when the epithelial function of cancer cells evolves to mesenchymal cell properties. Ovarian cancer cells separate from their primary sites of genesis (the ovary and/or fallopian tube) during the process of peritoneal migration. These malignant cells then invade the peritoneal molecular signal to reach specific organs and develop a secondary tumor.62 Studies show that tumor-derived exosomes (TEXs) play a pivotal role in the development of the premetastatic environment and cancer metastasis.84 Later on, discoveries stated that the creation of premetastatic environments is triggered by exosome integrins (ITGs), the key molecule that led to organ-specific metastasis.85 Integrins constructed by two subunits such as α and β and the combination of both subunits can guide circulated cancer cells in different destinations (bone, lung, liver brain, and lymph node)85,86 (Figure 5). An experiment was performed on human peritoneal mesothelial cells using Western blot, which showed overexpression of the α5β1-integrin (ITGA5B1) and colocalization with cysteine protease, suggesting the importance of the α5β1-integrin in ovarian cancer metastasis.87 A study conducted with orthotropic xenografted mice was done to conclude which integrin and molecular network epithelial ovarian cancer follows. The result stated that the IGF1R-6 integrin S100A4 network plays a pivotal role in the reoccurrence of EOC.88 Another study was conducted for high-grade serous ovarian cancer where it showed that the function of the α4 integrin was changed during metastasis.89 It is possible that TEX integrin expression will target a potential therapeutic strategy for metastatic OC in the future.
Figure 5.
Exosome-mediated organ-specific metastasis. (a) Circulated tumor cells migrate in different organs via the guidance of the exosome integrin. (b) Integrin contract by two subunits α and β. Created with BioRender.com.
8. TEXs Lead to Ovarian Cancer Therapy and Drug Resistance
TEXs of OC actively participate in the development of therapy and drug resistance. The internal cargo of TEXs regulates the event. A study conducted by Magee et al. concluded that miR-214 has an important role to play in cisplatin resistance in a few specific types of ovarian cancer.90 In ovarian cancer, adiposity and cancer-associated fibroblast-derived exosome miRNA-21 result in lower sensitivity of OC cells to the drug paclitaxel.91 A scientific study suggests that the ovarian cancer TEX precursor phage miRNA-21–5p induces drug resistance to cisplatin by downregulating the NAV3 gene.92 TEXs miRNA and lncRNA play a vital role in inducing drug resistance. Studies reveal that lnc MALAT1 and LINCO1118 prompt upregulation of ABCC1 which then helps in drug resistance.93,94 The presence of a protein-coding gene, Amphiregulin (AREG), plays a vital role in ovarian cancer stemness. Studies reveal that AREG also plays an important role in resisting drugs by taking up the AREG-EGFR-ERK pathway.95 Epigenetics investigation suggests that E2F6 ceRNA inhibited miRNA193a, which resulted in the promotion of ovarian cancer stemness.96 In OC, a tumor microenvironment associated macrophage derives exosome miRNA-223 to develop chemoresistance.97 Exosomes are also involved in radioresistant development.98
9. TEXs Are a Source of Ovarian Cancer Biomarkers
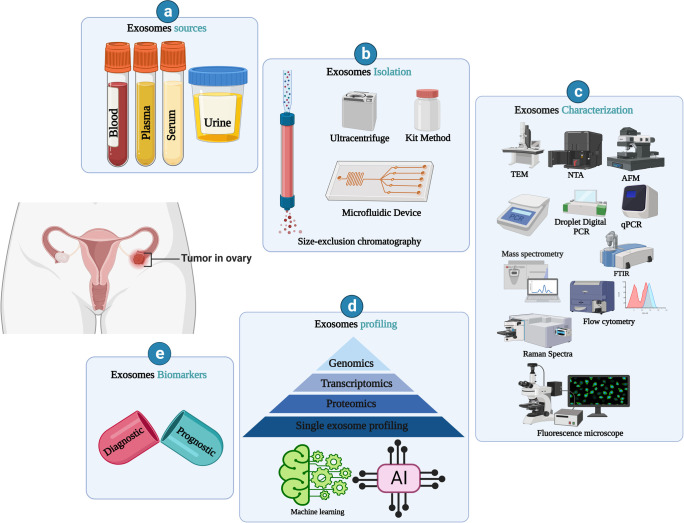
Exosomes are a promising source of cancer biomarkers.99−102 In OC, it supports early detection of it. Exosome association in OC biomarker research is explained in Figure 6. Body fluid circulated exosomes help overcome the limitation of several barriers and showed promising OC screening in the early stage.103,13,15 Exosome cargo, such as proteins, cell surface receptors, and miRNAs, is the major focus in exosome-based biomarker development. OC TEXs are capable of transforming normal cells into cancerous cells. Exosomes from ovarian cancer contain an increased level of some types of protein (CD24 and Claudin-4).104 Small heat shock proteins (HSPs) are another potential biomarker present in the serum of ovarian cancer patients.45
Figure 6.
Exosome-based ovarian cancer biomarkers. (a) Ovarian cancer biomarker source (blood, plasma, serum, and urine). (b) Isolation, (c) analysis tool, (d) exosome profiling, and (e) exosomes biomarkers. Created with BioRender.com.
Ascites of ovarian cancer contain an exosome proteome that is used in monitoring the therapeutic response. The elevated expression of exosome-derived plasma gelsolin (Ex-pGSN) is a prognostic marker of the poor survival of patients of ovarian cancer.105 There are also OC drug-resistant cancer cell derived exosome-associated molecular signatures of OC drug resistance development. Chemoresistance-related molecules such as annexin A3, MRP2, ATP7A, and ATP are expressed here, which indicates the effectiveness of chemotherapy methods. The microfluidic device is used for the isolation of exosomes for the advanced stage of OC biomarker investigation.106 Exosome-based biomarkers are indicators of the therapeutic outcome in OC. Exosome-associated biomarkers are classified into two major classes, diagnostic and prognostic. These are indicators of several cellular changes during OC development.107 Exosome-derived miR-6126 link with chemoresistance development in OC.108 Plasma exosomes derive miRNA-320, miRNA-200, miRNA-21, and miRNA-100 as early diagnostic biomarkers of ovarian cancer. Exosome miRNA-associated biomarker investigation is the most challenging because of the molecular instability and exosome heterogenicity.109,110 Hence, exosomes are the mastermind of aggressive OC development, so efficient instrumental development and single exosome profiling110,111 support efficient biomarker development. Further extensive clinical studies with exosomes could make a breakthrough in the medical field. Several exosome-based OC biomarkers are listed in Table 2.
Table 2. Exosome-Based Biomarkers of Ovarian Cancer.
| Ovarian cancer biomarker | Exosome Source | Exosome molecule | Clinical impact | References |
|---|---|---|---|---|
| Diagnostic marker | Plasma | miRNA-320 | Higher expression involved in epithelial ovarian cancer | (103) |
| Serum | miRNA-21, miRNA-141, miRNA-200a, miRNA-200c, miR-200b, miRNA-203, miRNA-205, miRNA-214 | This group of miRNA is highly expressed compared to the healthy individual | (13) | |
| Urine | RNA-30a-5p | Ovarian cancer cells derived higher expression of this miRNA as a diagnostic biomarker | (15) | |
| Prognostic marker | Plasma | let-7f (miRNA) | Lower expression indicates low recovery | (112) |
| Serum | aHIF (lncRNA) | Expression level is high in ovarian cancer | (113) | |
| Urine | RNA-30a-5p | Lower expression | (15) |
10. Exosome-Based Therapeutic Strategies for Ovarian Cancer
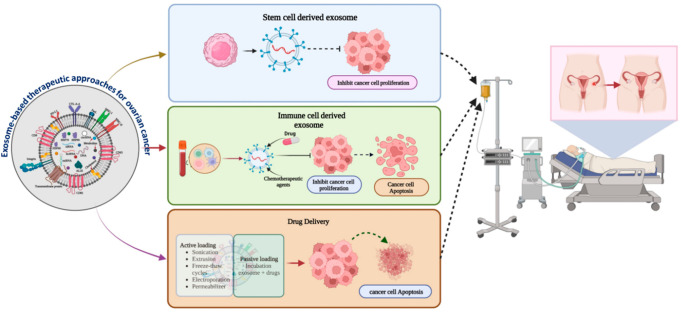
Exosome-based cancer healing is a potential approach.114−118 The therapeutic impact of exosomes in OC is summarized in Figure 7. It is a smart drug delivery tool for cancer.61,16 Exosomes overcome the toxicity limitations of several traditional approaches. It shows that integrin and tetraspanin expressive proteins control cell-specific drug transport.119 Through the application of advanced nanotechnology, exosomes deliver multiple therapeutic cargos such as water-soluble drugs, micro-RNA (miRNA), and small interfering RNA (siRNA), to ovarian cancer cell sites.120 Transfection gene products like miRNA-143, miRNA-146b, and let-7a are packaged into the exosome lumen, which inhibits ovarian cancer growth. Dendritic cells derive exosomes that have an important role in the development of an antitumor response.121 This activation cascade interlinks with the NK cells and T-cell-mediated antitumor cytotoxic activity in the immune system with higher expression of the MHC molecule. Research data show that the combination of exosomes and the granulocyte-macrophage colony-stimulating factor (GM-CSF) elevates the antitumor response via cytotoxic T-cell (Tc, CD8+ is the major cell population work against cancer) activation.18 This is a potential exosome-based immune therapeutic approach. Exosome-based miRNA-484 transportation in OC cells develops more chemotherapy sensitivity (via reducing the expression of VEGR-A).14 Stem cell derived exosome miRNA reduces OC cell proliferation.122 Lemon-derived exosome-mediated anticancer drug delivery in OC shows promising results.123 The engineered exosome is a new platform for the development of exosome-based cancer therapies (the more specific way it works).62 The chimeric antigen receptor T cell (CAR-T) derived exosome is an innovative initiation of CAR-T technology because it overcomes the limitation of toxicity (it develops acute inflammation) of CAR-T cell therapy, but the CAR-T cell-derived exosome is less toxic than CAR-T cell therapy.124,125 We hope that exosomes will become a smart solution for ovarian cancer in the future. Exosome-based cancer therapeutic applications require more clinical and toxicological investigation for affordable and efficient cancer therapy development.
Figure 7.
Exosome-based therapeutic approaches for ovarian cancer. Created with BioRender.com.
11. Clinical Trials
Exosomes and ovarian cancer are associated with several clinical studies conducted globally. A clinical investigation is underway in China to understand the role of exosome-derived noncoding RNA in the development of ovarian cancer. This work is also dedicated to the development of noncoding RNA-based diagnostic and prognostic biomarkers for ovarian cancer. The National Institute of Health (NIH) funded a clinical investigation report that showed blood-circulated exosome and monocyte crosstalk in anticancer activity in ovarian cancer, epithelial ovarian cancer, and fallopian tube cancer. Another clinical trial based on the exosome express heat shock protein 70 (HSP70) is being conducted in France in patients with ovarian cancer. In the future, a more clinical investigation need next-generation exosome-based ovarian cancer theranostic development. Clinical trials are listed in Table 3.
Table 3. Exosome-Based Clinical Trial in Ovarian Cancer.
| Trail ID | Status | Timeline | Core study | Clinical impact | Funding |
|---|---|---|---|---|---|
| NCT03738319 | Unknowna | 2018–2019 | Sequencing of miRNA/lncRNA | Study based on noncoding RNA of exosome, role in epithelial ovarian cancer and diagnosis, and prognosis marker development | Professor Lei Li, Peking Union Medical College Hospital, China |
| NCT02063464 | Completed | 2014–2016 | Ovarian cancer ovarian, epithelial, and fallopian tube cancer patients’ blood | Monocytes’ anticancer activity and exosome interlink | NIH, USA |
| NCT02662621 | Completed | 2015–2019 | Isolation of exosomes from blood and urine | Exosome HSP70 protein role in ovarian cancer and earlier detection of the biomarker of ovarian cancer | Centre Georges Francois Leclerc, France |
The study has passed its completion date, and the status has not been verified in more than two years (source: https://clinicaltrials.gov/).
12. Exploring Tomorrow’s Horizons: Envisioning the Future of Cancer Research
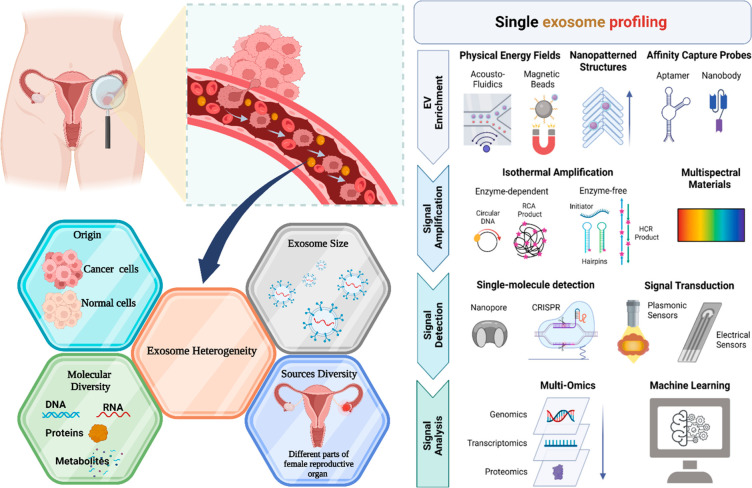
Exosome-based cancer research faces several critical questions that require in-depth exploration, including (a) standard isolation protocols, (b) exosome heterogeneity,110 (c) therapeutic exosome toxicity, and (d) comprehensive study of molecular components of exosomes. The complexity of exosome heterogeneity arises from factors such as origin, size, molecular diversity, and source diversity.110 In the context of ovarian cancer, blood-circulating exosomes represent a mixture of cell-secreted exosomes from various parts of the female reproductive system. To effectively address these challenges, a single exosome profiling approach (Figure 8) is proposed, encompassing exosome isolation and downstream profiling. Techniques such as microfluidic devices, magnetic bead-associated methods,5 and aptamer-based affinity principles for isolation126 can be employed. Exosome molecular cargo profiling can be achieved through various molecular approaches,127 including nanopore-based detection for individual molecular signaling128 and CRISPR-based sensors for surface protein analysis in cancer.129 Classifying specific exosome subpopulations can be facilitated by plasmonic sensors130 and electrochemical sensors.5 The final stage of single exosome profiling incorporates multiple omics approaches (genomic, transcriptomic, and proteomic)131 and machine learning132 to identify specific cancer biomarkers. This comprehensive profiling process brings us closer to achieving precision and personalized medicine in cancer treatment.131 Toxicological profiling of exosomes represents another essential aspect of exosome research. Exosome secretion patterns and the presence of internal cargo can alter the behavior of the recipient cell when exposed to various components such as drugs and chemicals.133 While current research suggests that mesenchymal stem cell (MSC)-derived exosomes are nontoxic134 and various exosome sources possess therapeutic potential (e.g., plant exosomes135 and dendritic-cell-derived exosomes), further in-depth toxicology profiling research is necessary. Additionally, the establishment of standardized protocols for exosome downstream applications is crucial. Overcoming these barriers will usher in a new era of exosome-based cancer vaccines.136 Exosome-based cancer research supports the reach of a precision medicine era.
Figure 8.
Exosome heterogeneity. (a) Exosome heterogeneity serrulate several facts. (b) Single exosome profiling. Adapted with permission from ref (110). Copyright 2022. American Chemical Society. Created with BioRender.com.
13. Unraveling the Enigma of Ovarian Cancer: Harnessing the Power of Exosomes for a Brighter Tomorrow
The extraordinary potential of exosome-based theranostics in revolutionizing the diagnosis and treatment of ovarian cancer metastasis is indisputable. Exploiting the distinctive properties and communicative capabilities of exosomes enables researchers to uncover invaluable insights into the intricacies of cancer progression, metastasis, and drug resistance. While challenges remain in aspects such as exosome heterogeneity, isolation, toxicity, and molecular profiling, the unyielding pursuit of knowledge and technological advancements propel us toward overcoming these obstacles. In turn, we advance closer to realizing the full potential of exosomes as formidable allies in combating ovarian cancer. The significant clinical implications of exosome-based ovarian cancer screening establish a solid foundation for more efficacious early detection and intervention approaches. A comprehensive understanding of exosome biology is critical to ensure the safety and efficacy of exosome-based theragnostics while mitigating potential health risks. By bridging the gap between our current knowledge and the untapped potential of exosome-based theranostics, we are venturing into a transformative age of precision and personalized medicine. This paradigm shift is poised to reshape our approach to ovarian cancer metastasis, instilling hope for more precise interventions, enhanced patient outcomes, and the development of new, innovative cancer vaccines. Delving into the enigma of exosomes and their potential applications reveals a world of possibilities for next-generation cancer therapy. With unwavering optimism and commitment, the future of exosome research is primed to inaugurate a ground-breaking era of precision and personalized medicine, ultimately culminating in the invention of cutting-edge cancer vaccine solutions.
Acknowledgments
The authors would like to acknowledge their respective departments for the conduct of the study.
Data Availability Statement
All relevant data are within the manuscript.
The authors declare no competing financial interest.
References
- Sung H.; Ferlay J.; Siegel R. L.; Laversanne M.; Soerjomataram I.; Jemal A.; et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–49. 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Huang J.; Chan W. C.; Ngai C. H.; Lok V.; Zhang L.; Lucero-Prisno D. E. 3rd; Xu W.; Zheng Z. J.; Elcarte E.; Withers M.; Wong M. C. S. On Behalf Of Ncd Global Health Research Group Of Association Of Pacific Rim Universities Apru. Worldwide Burden, Risk Factors, and Temporal Trends of Ovarian Cancer: A Global Study. Cancers (Basel). 2022, 14, 2230. 10.3390/cancers14092230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondelli V.; Helmy S.; Passignani G.; Parisse P.; Di Silvestre D. Integrated Strategies for a Holistic View of Extracellular Vesicles. ACS Omega. 2022, 7, 19058–19069. 10.1021/acsomega.2c01003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft P. K.; Sharma S.; Godbole N.; Rice G. E.; Salomon C. Ovarian-Cancer-Associated Extracellular Vesicles: Microenvironmental Regulation and Potential Clinical Applications. Cells. 2021, 10, 2272. 10.3390/cells10092272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao H.; Im H.; Castro C. M.; Breakefield X.; Weissleder R.; Lee H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–50. 10.1021/acs.chemrev.7b00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L.; Ling W.; Ruan Z. TAM-derived extracellular vesicles containing microRNA-29a-3p explain the deterioration of ovarian cancer. Mol. Ther Nucleic Acids. 2021, 25, 468–82. 10.1016/j.omtn.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J. J.; Lin X. J.; Tang X. Y.; Zheng T. T.; Lin Y. Y.; Hua K. Q. Exosomal Metastasis-Associated Lung Adenocarcinoma Transcript 1 Promotes Angiogenesis and Predicts Poor Prognosis in Epithelial Ovarian Cancer. Int. J. Biol. Sci. 2018, 14, 1960–73. 10.7150/ijbs.28048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czystowska-Kuzmicz M.; Sosnowska A.; Nowis D.; Ramji K.; Szajnik M.; Chlebowska-Tuz J.; et al. Small extracellular vesicles containing arginase-1 suppress T-cell responses and promote tumor growth in ovarian carcinoma. Nat. Commun. 2019, 10, 3000. 10.1038/s41467-019-10979-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.; Zhang X.; Wang J.; Li M.; Cao C.; Tan J.; et al. TGFbeta1 in fibroblasts-derived exosomes promotes epithelial-mesenchymal transition of ovarian cancer cells. Oncotarget. 2017, 8, 96035–47. 10.18632/oncotarget.21635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W.; Lei N.; Zhou J.; Chen M.; Guo R.; Qin B.; Li Y.; Chang L.; et al. Extracellular vesicles in ovarian cancer chemoresistance, metastasis, and immune evasion. Cell Death Dis. 2022, 13, 64. 10.1038/s41419-022-04510-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Wei Y. J.; Zhang Y. F.; Liu H. W.; Zhang Y. F. Emerging Functions and Clinical Applications of Exosomal ncRNAs in Ovarian Cancer. Front Oncol. 2021, 11, 765458. 10.3389/fonc.2021.765458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runz S.; Keller S.; Rupp C.; Stoeck A.; Issa Y.; Koensgen D.; et al. Malignant ascites-derived exosomes of ovarian carcinoma patients contain CD24 and EpCAM. Gynecol Oncol. 2007, 107, 563–71. 10.1016/j.ygyno.2007.08.064. [DOI] [PubMed] [Google Scholar]
- Taylor D. D.; Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008, 110, 13–21. 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Alharbi M.; Sharma S.; Guanzon D.; Lai A.; Zuniga F.; Shiddiky M. J. A.; et al. miRNA signature in small extracellular vesicles and their association with platinum resistance and cancer recurrence in ovarian cancer. Nanomedicine. 2020, 28, 102207. 10.1016/j.nano.2020.102207. [DOI] [PubMed] [Google Scholar]
- Zhou J.; Gong G.; Tan H.; Dai F.; Zhu X.; Chen Y.; Wang J.; Liu Y.; Chen P.; Wu X.; Wen J. Urinary microRNA-30a-5p is a potential biomarker for ovarian serous adenocarcinoma. Oncol. Rep. 2015, 33, 2915–23. 10.3892/or.2015.3937. [DOI] [PubMed] [Google Scholar]
- Kar R.; Dhar R.; Mukherjee S.; Nag S.; Gorai S.; Mukerjee N.; Mukherjee D.; Vatsa R.; Chandrakanth Jadhav M.; Ghosh A.; Devi A.; Krishnan A.; Thorat N. D. Exosome-Based Smart Drug Delivery Tool for Cancer Theranostics. ACS Biomater Sci. Eng. 2023, 9, 577–594. 10.1021/acsbiomaterials.2c01329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giusti I.; Di Francesco M.; D’Ascenzo S.; Palmerini M. G.; Macchiarelli G.; Carta G.; et al. Ovarian cancer-derived extracellular vesicles affect normal human fibroblast behavior. Cancer Biol. Ther. 2018, 19, 722–734. 10.1080/15384047.2018.1451286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G.; Huang A. C.; Zhang W.; Zhang G.; Wu M.; Xu W.; Yu Z.; Yang J.; Wang B.; Sun H.; Xia H.; Man Q.; Zhong W.; Antelo L. F.; Wu B.; Xiong X.; Liu X.; Guan L.; Li T.; Liu S.; Yang R.; Lu Y.; Dong L.; McGettigan S.; Somasundaram R.; Radhakrishnan R.; Mills G.; Lu Y.; Kim J.; Chen Y. H.; Dong H.; Zhao Y.; Karakousis G. C.; Mitchell T. C.; Schuchter L. M.; Herlyn M.; Wherry E. J.; Xu X.; Guo W. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018, 560, 382–386. 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegmans J. P.; Gerber P. J.; Lambrecht B. N. Exosomes. Methods Mol. Biol. 2008, 484, 97–109. 10.1007/978-1-59745-398-1_7. [DOI] [PubMed] [Google Scholar]
- Shivji G. G.; Dhar R.; Devi A. Role of exosomes and its emerging therapeutic applications in the pathophysiology of non-infectious diseases. Biomarkers. 2022, 27, 534–548. 10.1080/1354750X.2022.2067233. [DOI] [PubMed] [Google Scholar]
- Bhattacharya B.; Dhar R.; Mukherjee S.; et al. Exosome DNA: An untold story of cancer. Clin Transl Disc. 2023, 3, e218 10.1002/ctd2.218. [DOI] [Google Scholar]
- Li C.; Zhou T.; Chen J.; Li R.; Chen H.; Luo S.; et al. The role of Exosomal miRNAs in cancer. Journal of Translational Medicine. 2022, 20, 6. 10.1186/s12967-021-03215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongsma M. L.; Berlin I.; Wijdeven R. H.; Janssen L.; Janssen G. M.; Garstka M. A.; et al. An ER-Associated Pathway Defines Endosomal Architecture for Controlled Cargo Transport. Cell. 2016, 166, 152–66. 10.1016/j.cell.2016.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S.; Dhar R.; Gurudas Shivji G.; Dey D.; Devi A.; Jha S. K.; Adhikari M. D.; Gorai S. Clinical Impact of Exosomes in Colorectal Cancer Metastasis. ACS Appl. Bio Mater. 2023, 6, 2576. 10.1021/acsabm.3c00199. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Liu Y.; Liu H.; Tang W. H. Exosomes: biogenesis, biologic function and clinical potential. Cell & bioscience. 2019, 9, 19. 10.1186/s13578-019-0282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boura E.; Ivanov V.; Carlson L. A.; Mizuuchi K.; Hurley J. H. Endosomal sorting complex required for transport (ESCRT) complexes induce phase-separated microdomains in supported lipid bilayers. J. Biol. Chem. 2012, 287, 28144–51. 10.1074/jbc.M112.378646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R.; Mallik S.; Devi A. Exosomal microRNAs (exoMIRs): micromolecules with macro impact in oral cancer. 3 Biotechnol. 2022, 12, 155. 10.1007/s13205-022-03217-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R.; Mukherjee S.; Mukerjee N.; Mukherjee D.; Devi A.; Ashraf G. M.; Alserihi R. F.; Tayeb H. H.; Hashem A. M.; Alexiou A.; Thorate N. Interrelation between extracellular vesicles miRNAs with chronic lung diseases. J. Cell Physiol. 2022, 237, 4021–4036. 10.1002/jcp.30867. [DOI] [PubMed] [Google Scholar]
- Kim Y. S.; Ahn J. S.; Kim S.; Kim H. J.; Kim S. H.; Kang J. S. The potential theragnostic (diagnostic+therapeutic) application of exosomes in diverse biomedical fields. Korean J. Physiol Pharmacol. 2018, 22, 113–125. 10.4196/kjpp.2018.22.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne W. M.; Buchkovich N. J.; Emr S. D. The ESCRT pathway. Dev Cell. 2011, 21, 77–91. 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- van Niel G.; Charrin S.; Simoes S.; Romao M.; Rochin L.; Saftig P.; Marks M. S.; Rubinstein E.; Raposo G. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell. 2011, 21, 708–721. 10.1016/j.devcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S.; Dhar R.; Jonnalagadda S.; Gorai S.; Nag S.; Kar R.; Mukerjee N.; Mukherjee D.; Vatsa R.; Arikketh D.; Krishnan A.; Gundamaraju R.; Jha S. K.; Alexiou A.; Papadakis M. Exosomal miRNAs and breast cancer: a complex theranostics interlink with clinical significance. Biomarkers. 2023, 1–17. 10.1080/1354750X.2023.2229537. [DOI] [PubMed] [Google Scholar]
- Whiteside T. L. The effect of tumor-derived exosomes on immune regulation and cancer immunotherapy. Future oncology (London, England). 2017, 13, 2583–92. 10.2217/fon-2017-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Tang M. Exosomes released from M2 macrophages transfer miR-221–3p contributed to EOC progression through targeting CDKN1B. Cancer medicine. 2020, 9, 5976–88. 10.1002/cam4.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W.; Dean D. C.; Hornicek F. J.; Shi H.; Duan Z. Exosomes promote pre-metastatic niche formation in ovarian cancer. Molecular cancer. 2019, 18, 124. 10.1186/s12943-019-1049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak M.; Klink M. The Role of Tumor-Associated Macrophages in the Progression and Chemoresistance of Ovarian Cancer. Cells. 2020, 9, 1299. 10.3390/cells9051299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng P.; Yan Y.; Keng S. Exosomes in the ascites of ovarian cancer patients: origin and effects on anti-tumor immunity. Oncol. Rep. 2011, 25, 749–762. 10.3892/or.2010.1119. [DOI] [PubMed] [Google Scholar]
- Labani-Motlagh A.; Israelsson P.; Ottander U.; Lundin E.; Nagaev I.; Nagaeva O.; Dehlin E.; Baranov V.; Mincheva-Nilsson L. Differential expression of ligands for NKG2D and DNAM-1 receptors by epithelial ovarian cancer-derived exosomes and its influence on NK cell cytotoxicity. Tumour Biol. 2016, 37, 5455–66. 10.1007/s13277-015-4313-2. [DOI] [PubMed] [Google Scholar]
- Li X.; Liu Y.; Zheng S.; Zhang T.; Wu J.; Sun Y.; Zhang J.; Liu G. Role of exosomes in the immune microenvironment of ovarian cancer. Oncol Lett. 2021, 21, 377. 10.3892/ol.2021.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. D.; Gerçel-Taylor C. Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects. British journal of cancer. 2005, 92, 305–11. 10.1038/sj.bjc.6602316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy G. N.; Loyall J.; Berenson C. S.; Kelleher R. J. Jr; Iyer V.; Balu-Iyer S. V.; et al. Sialic Acid-Dependent Inhibition of T Cells by Exosomal Ganglioside GD3 in Ovarian Tumor Microenvironments. Journal of immunology (Baltimore, Md: 1950). 2018, 201, 3750–8. 10.4049/jimmunol.1801041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy G. N.; Loyall J.; Maguire O.; Iyer V.; Kelleher R. J. Jr; Minderman H.; et al. Exosomes Associated with Human Ovarian Tumors Harbor a Reversible Checkpoint of T-cell Responses. Cancer immunology research. 2018, 6, 236–47. 10.1158/2326-6066.CIR-17-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S.; König A. K.; Marmé F.; Runz S.; Wolterink S.; Koensgen D.; et al. Systemic presence and tumor-growth promoting effect of ovarian carcinoma released exosomes. Cancer letters. 2009, 278, 73–81. 10.1016/j.canlet.2008.12.028. [DOI] [PubMed] [Google Scholar]
- Chang Y. H.; Lin Y. J.; Huang C. Y.; Harnod T.; Ding D. C. Shikonin impedes type 2 ovarian cancer progression via FasL/caspase-8 and mir-874–3p/XIAP axis and prohibits the properties of stemness. American J. Cancer Res. 2022, 12, 4584–4601. [PMC free article] [PubMed] [Google Scholar]
- Wyciszkiewicz A.; Kalinowska-Łyszczarz A.; Nowakowski B.; Kazmierczak K.; Osztynowicz K.; Michalak S. Expression of small heat shock proteins in exosomes from patients with gynecologic cancers. Scientific reports. 2019, 9, 9817. 10.1038/s41598-019-46221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbo J.; Marcion G.; Cordonnier M.; Dias A. M. M.; Pernet N.; Hammann A.; et al. Restoring Anticancer Immune Response by Targeting Tumor-Derived Exosomes With a HSP70 Peptide Aptamer. Journal of the National Cancer Institute. 2016, 108, 330. 10.1093/jnci/djv330. [DOI] [PubMed] [Google Scholar]
- Hu Y.; Li D.; Wu A.; Qiu X.; Di W.; Huang L.; et al. TWEAK-stimulated macrophages inhibit metastasis of epithelial ovarian cancer via exosomal shuttling of microRNA. Cancer letters. 2017, 393, 60–7. 10.1016/j.canlet.2017.02.009. [DOI] [PubMed] [Google Scholar]
- Dongre A.; Weinberg R. A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. 10.1038/s41580-018-0080-4. [DOI] [PubMed] [Google Scholar]
- Tkach M.; Théry C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell. 2016, 164, 1226–32. 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- Croft P. K.; Sharma S.; Godbole N.; Rice G. E.; Salomon C. Ovarian-Cancer-Associated Extracellular Vesicles: Microenvironmental Regulation and Potential Clinical Applications. Cells. 2021, 10, 2272. 10.3390/cells10092272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino J. S.; Akhter T.; Bravo-Cordero J. J. Remodeling the ECM: Implications for Metastasis and Tumor Dormancy. Cancers 2021, 13, 4916. 10.3390/cancers13194916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios-Ferrer J. L.; García-Ortega M. B.; Gallardo-Gómez M.; García M.; Díaz C.; Boulaiz H.; et al. Metabolomic profile of cancer stem cell-derived exosomes from patients with malignant melanoma. Mol. Oncol. 2021, 15, 407–28. 10.1002/1878-0261.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard E. T.; Bozic I.; Riddell S. R.; Ghajar C. M. Dormant tumour cells, their niches and the influence of immunity. Nat. Cell Biol. 2018, 20, 1240–1249. 10.1038/s41556-018-0214-0. [DOI] [PubMed] [Google Scholar]
- Kumari S.; Patro A. R. K.; Mishra B.; Jena S. K.; Singh S. Serum Lysyl Oxidase Levels and Lysyl Oxidase Gene Polymorphism in Ovarian Cancer Patients of Eastern Indian Population. Diagnostics (Basel, Switzerland). 2022, 12, 53. 10.3390/diagnostics12010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet S. D.; Ricard-Blum S. Lysyl oxidases: from enzyme activity to extracellular matrix cross-links. Essays in biochemistry. 2019, 63, 349–64. 10.1042/EBC20180050. [DOI] [PubMed] [Google Scholar]
- Conklin M. W.; Keely P. J. Why the stroma matters in breast cancer: insights into breast cancer patient outcomes through the examination of stromal biomarkers. Cell adhesion & migration. 2012, 6, 249–60. 10.4161/cam.20567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.; Zhao L.; Rojas C.; Bateman N. W.; Yao H.; Lara O. D.; Celestino J.; Morgan M. B.; Nguyen T. V.; Conrads K. A.; Rangel K. M.; Dood R. L.; Hajek R. A.; Fawcett G. L.; Chu R. A.; Wilson K.; Loffredo J. L.; Viollet C.; Jazaeri A. A.; Dalgard C. L.; Mao X.; Song X.; Zhou M.; Hood B. L.; Banskota N.; Wilkerson M. D.; Te J.; Soltis A. R.; Roman K.; Dunn A.; Cordover D.; Eterovic A. K.; Liu J.; Burks J. K.; Baggerly K. A.; Fleming N. D.; Lu K. H.; Westin S. N.; Coleman R. L.; Mills G. B.; Casablanca Y.; Zhang J.; Conrads T. P.; Maxwell G. L.; Futreal P. A.; Sood A. K. Molecular Analysis of Clinically Defined Subsets of High-Grade Serous Ovarian Cancer. Cell Rep. 2020, 31, 107502. 10.1016/j.celrep.2020.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi M.; Dehghani S.; Nosrati R.; Ghanei M.; Salmaninejad A.; Rajaie S.; et al. Current insights into the metastasis of epithelial ovarian cancer - hopes and hurdles. Cellular oncology (Dordrecht). 2020, 43, 515–38. 10.1007/s13402-020-00513-9. [DOI] [PubMed] [Google Scholar]
- Bortot B.; Apollonio M.; Rampazzo E.; Valle F.; Brucale M.; Ridolfi A.; et al. Small extracellular vesicles from malignant ascites of patients with advanced ovarian cancer provide insights into the dynamics of the extracellular matrix. Mol. Oncol. 2021, 15, 3596–614. 10.1002/1878-0261.13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J.; Zhu X.; Fei J.; Shi P.; Yu S.; Zhou J. Advances of exosome in the development of ovarian cancer and its diagnostic and therapeutic prospect. OncoTargets and therapy. 2018, 11, 2831–41. 10.2147/OTT.S159829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu A.; Sawada K.; Kimura T. Pathophysiological Role and Potential Therapeutic Exploitation of Exosomes in Ovarian Cancer. Cells. 2020, 9, 814. 10.3390/cells9040814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K.; Sawada K.; Kobayashi M.; Miyamoto M.; Shimizu A.; Yamamoto M.; Kinose Y.; Kimura T.; et al. Role of the Exosome in Ovarian Cancer Progression and Its Potential as a Therapeutic Target. Cancers. 2019, 11, 1147. 10.3390/cancers11081147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B.; Peng P.; Chen S.; Li L.; Zhang M.; Cao D.; et al. Characterization and proteomic analysis of ovarian cancer-derived exosomes. Journal of proteomics. 2013, 80, 171–82. 10.1016/j.jprot.2012.12.029. [DOI] [PubMed] [Google Scholar]
- Yi H.; Ye J.; Yang X. M.; Zhang L. W.; Zhang Z. G.; Chen Y. P. High-grade ovarian cancer secreting effective exosomes in tumor angiogenesis. Int. J. Clin. Experimental Pathology 2015, 8, 5062–5070. [PMC free article] [PubMed] [Google Scholar]
- Chen X.; Ying X.; Wang X.; Wu X.; Zhu Q.; Wang X. Exosomes derived from hypoxic epithelial ovarian cancer deliver microRNA-940 to induce macrophage M2 polarization. Oncol. Rep. 2017, 38, 522–8. 10.3892/or.2017.5697. [DOI] [PubMed] [Google Scholar]
- Bretz N. P.; Ridinger J.; Rupp A. K.; Rimbach K.; Keller S.; Rupp C.; et al. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via Toll-like receptor signaling. J. Biol. Chem. 2013, 288, 36691–702. 10.1074/jbc.M113.512806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A.; Sawada K.; Nakamura K.; Kinose Y.; Nakatsuka E.; Kobayashi M.; et al. Exosomal miR-99a-5p is elevated in sera of ovarian cancer patients and promotes cancer cell invasion by increasing fibronectin and vitronectin expression in neighboring peritoneal mesothelial cells. BMC cancer. 2018, 18, 1065. 10.1186/s12885-018-4974-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X.; Shen H.; Yin X.; Yang M.; Wei H.; Chen Q.; et al. Macrophages derived exosomes deliver miR-223 to epithelial ovarian cancer cells to elicit a chemoresistant phenotype. Journal of experimental & clinical cancer research: CR. 2019, 38, 81. 10.1186/s13046-019-1095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R.; Devi A.; Gorai S.; Jha S. K.; Alexiou A.; Papadakis M. Exosome and epithelial-mesenchymal transition: A complex secret of cancer progression. J. Cell Mol. Med. 2023, 27, 1603–1607. 10.1111/jcmm.17755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai S.; Wei Y.; Liu R.; Xu R.; Xiang L.; Du J. Role of tumour-derived exosomes in metastasis. Biomedicine Pharmacotherapy 2022, 147, 112657. 10.1016/j.biopha.2022.112657. [DOI] [PubMed] [Google Scholar]
- Roma-Rodrigues C.; Mendes R.; Baptista P. V.; Fernandes A. R. Targeting Tumor Microenvironment for Cancer Therapy. Int. J. Mol. Sci. 2019, 20 (4), 840. 10.3390/ijms20040840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M.; Sawada K.; Kimura T. Potential of Integrin Inhibitors for Treating Ovarian Cancer: A Literature Review. Cancers 2017, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto E.; Park E. J.; Shimaoka M. Methods to Study Integrin Functions on Exosomes. Methods Mol. Biol. 2021, 2217, 265–81. 10.1007/978-1-0716-0962-0_15. [DOI] [PubMed] [Google Scholar]
- Valdembri D.; Serini G. The roles of integrins in cancer. Faculty reviews. 2021, 10, 45. 10.12703/r/10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghafouri-Fard S.; Shoorei H.; Taheri M. miRNA profile in ovarian cancer. Experimental and molecular pathology. 2020, 113, 104381. 10.1016/j.yexmp.2020.104381. [DOI] [PubMed] [Google Scholar]
- Flores C. P.; Garcia-Vazquez R.; Rincon D. G.; Ruiz-Garcia E.; De La Vega H. A.; Marchat L. A.; Salinas Vera Y. M.; Lopez-Camarillo C.; et al. MicroRNAs driving invasion and metastasis in ovarian cancer: Opportunities for translational medicine (Review). International journal of oncology. 2017, 50, 1461–1476. 10.3892/ijo.2017.3948. [DOI] [PubMed] [Google Scholar]
- Chen S. N.; Chang R.; Lin L. T.; Chern C. U.; Tsai H. W.; Wen Z. H.; et al. MicroRNA in Ovarian Cancer: Biology, Pathogenesis, and Therapeutic Opportunities. International journal of environmental research and public health. 2019, 16, 1510. 10.3390/ijerph16091510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb B.; Uddin A.; Chakraborty S. miRNAs and ovarian cancer: An overview. Journal of cellular physiology. 2018, 233, 3846–54. 10.1002/jcp.26095. [DOI] [PubMed] [Google Scholar]
- Loginov V. I.; Pronina I. V.; Burdennyy A. M.; Filippova E. A.; Kazubskaya T. P.; Kushlinsky D. N.; et al. Novel miRNA genes deregulated by aberrant methylation in ovarian carcinoma are involved in metastasis. Gene. 2018, 662, 28–36. 10.1016/j.gene.2018.04.005. [DOI] [PubMed] [Google Scholar]
- Panoutsopoulou K.; Avgeris M.; Scorilas A. miRNA and long non-coding RNA: molecular function and clinical value in breast and ovarian cancers. Expert review of molecular diagnostics. 2018, 18, 963–79. 10.1080/14737159.2018.1538794. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Tang D. Y.; Zuo X.; Zhang T. D.; Wang C. Identification of lncRNA-miRNA-mRNA regulatory network associated with epithelial ovarian cancer cisplatin-resistant. Journal of cellular physiology. 2019, 234, 19886–94. 10.1002/jcp.28587. [DOI] [PubMed] [Google Scholar]
- Mitra R.; Chen X.; Greenawalt E. J.; Maulik U.; Jiang W.; Zhao Z.; Eischen C. M.; et al. Decoding critical long non-coding RNA in ovarian cancer epithelial-to-mesenchymal transition. Nat. Commun. 2017, 8, 1604. 10.1038/s41467-017-01781-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J.; Gong L.; Li G.; Guo J.; Yi X.; Wang Z.; et al. Exosomes in ovarian cancer ascites promote epithelial-mesenchymal transition of ovarian cancer cells by delivery of miR-6780b-5p. Cell Death Dis. 2021, 12, 210. 10.1038/s41419-021-03490-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H.; Alečković M.; Lavotshkin S.; Matei I.; Costa-Silva B.; Moreno-Bueno G.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nature medicine. 2012, 18 (6), 883–91. 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A.; Costa-Silva B.; Shen T. L.; Rodrigues G.; Hashimoto A.; Tesic Mark M.; et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015, 527, 329–35. 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L.; Ma X.; Yu J. Exosomes and organ-specific metastasis. Mol. Ther Methods Clin Dev. 2021, 22, 133–147. 10.1016/j.omtm.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Tang M.; Zhu Q.; Wang X.; Lin Y.; Wang X. The exosomal integrin α5β1/AEP complex derived from epithelial ovarian cancer cells promotes peritoneal metastasis through regulating mesothelial cell proliferation and migration. Cellular oncology (Dordrecht). 2020, 43, 263–77. 10.1007/s13402-019-00486-4. [DOI] [PubMed] [Google Scholar]
- Deo A. N.; Thorat R.; Dhadve A. C.; De A.; Rekhi B.; Ray P. IGF1R-α6 integrin-S100A4 network governs the organ-specific metastasis of chemoresistant epithelial ovarian cancer cells. Biochimica et biophysica acta Molecular basis of disease. 2022, 1868, 166282. 10.1016/j.bbadis.2021.166282. [DOI] [PubMed] [Google Scholar]
- Krajnak S.; Jäkel J.; Anić K.; Schwab R.; Schmidt M.; Hasenburg A.; et al. Role of integrins in the metastatic spread of high-grade serous ovarian cancer. Archives of gynecology and obstetrics. 2022, 305, 1291–8. 10.1007/s00404-021-06281-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee P.; Shi L.; Garofalo M. Role of microRNAs in chemoresistance. Annals of translational medicine. 2015, 3, 332. 10.3978/j.issn.2305-5839.2015.11.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au Yeung C. L.; Co N. N.; Tsuruga T.; Yeung T. L.; Kwan S. Y.; Leung C. S.; et al. Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat. Commun. 2016, 7, 11150. 10.1038/ncomms11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pink R. C.; Samuel P.; Massa D.; Caley D. P.; Brooks S. A.; Carter D. R. The passenger strand, miR-21–3p, plays a role in mediating cisplatin resistance in ovarian cancer cells. Gynecol Oncol. 2015, 137, 143–51. 10.1016/j.ygyno.2014.12.042. [DOI] [PubMed] [Google Scholar]
- Shi C.; Wang M. LINC01118 Modulates Paclitaxel Resistance of Epithelial Ovarian Cancer by Regulating miR-134/ABCC1. Medical science monitor: international medical journal of experimental and clinical research. 2018, 24, 8831–9. 10.12659/MSM.910932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L.; Wang A.; Zhang Y.; Xu X.; Zhang X. Knockdown of MALAT1 enhances chemosensitivity of ovarian cancer cells to cisplatin through inhibiting the Notch1 signaling pathway. Experimental cell research. 2018, 366, 161–71. 10.1016/j.yexcr.2018.03.014. [DOI] [PubMed] [Google Scholar]
- Tung S. L.; Huang W. C.; Hsu F. C.; Yang Z. P.; Jang T. H.; Chang J. W.; et al. miRNA-34c-5p inhibits amphiregulin-induced ovarian cancer stemness and drug resistance via downregulation of the AREG-EGFR-ERK pathway. Oncogenesis. 2017, 6, e326 10.1038/oncsis.2017.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng F. H. C.; Lin H. Y.; Hwang T. W.; Chen Y. C.; Huang R. L.; Chang C. B.; et al. E2F6 functions as a competing endogenous RNA, and transcriptional repressor, to promote ovarian cancer stemness. Cancer science. 2019, 110, 1085–95. 10.1111/cas.13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X.; Shen H.; Yin X.; Yang M.; Wei H.; Chen Q.; et al. Macrophages derived exosomes deliver miR-223 to epithelial ovarian cancer cells to elicit a chemoresistant phenotype. J. Exp Clin Cancer Res. 2019, 38, 81. 10.1186/s13046-019-1095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque S.; Dhar R.; Kar R.; Mukherjee S.; Mukherjee D.; Mukerjee N.; Nag S.; Tomar N.; Mallik S. Cancer stem cells (CSCs): key player of radiotherapy resistance and its clinical significance. Biomarkers. 2023, 28, 139–151. 10.1080/1354750X.2022.2157875. [DOI] [PubMed] [Google Scholar]
- Wu T.; Liu Y.; Ali N. M.; Zhang B.; Cui X. Effects of Exosomes on Tumor Bioregulation and Diagnosis. ACS Omega. 2023, 8, 5157–5168. 10.1021/acsomega.2c06567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J.; Tan Z.; Zhang J.; An M.; Khaykin V. M.; Cuneo K. C.; Parikh N. D.; Lubman D. M. Sequential Method for Analysis of CTCs and Exosomes from the Same Sample of Patient Blood. ACS Omega. 2022, 7, 37581–37588. 10.1021/acsomega.2c04428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraoka S.; Hirano M.; Isoyama J.; Ishida M.; Tomonaga T.; Adachi J. Automated Proteomics Sample Preparation of Phosphatidylserine-Positive Extracellular Vesicles from Human Body Fluids. ACS Omega. 2022, 7, 41472–41479. 10.1021/acsomega.2c05244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie K.; Nanashima N.; Yokoyama Y.; Yoshioka H.; Watanabe J. Exosomal MicroRNA as Biomarkers for Diagnosing or Monitoring the Progression of Ovarian Clear Cell Carcinoma: A Pilot Study. Molecules (Basel, Switzerland). 2022, 27 (12), 3953. 10.3390/molecules27123953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan C.; Stevic I.; Müller V.; Ni Q.; Oliveira-Ferrer L.; Pantel K.; et al. Exosomal microRNAs as tumor markers in epithelial ovarian cancer. Mol. Oncol. 2018, 12, 1935–48. 10.1002/1878-0261.12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltész B.; Lukács J.; Szilágyi E.; Márton É; Szilágyi Bónizs M.; Penyige A.; et al. Expression of CD24 in plasma, exosome and ovarian tissue samples of serous ovarian cancer patients. Journal of biotechnology. 2019, 298, 16–20. 10.1016/j.jbiotec.2019.03.018. [DOI] [PubMed] [Google Scholar]
- Asare-Werehene M.; Nakka K.; Reunov A.; Chiu C. T.; Lee W. T.; Abedini M. R.; et al. The exosome-mediated autocrine and paracrine actions of plasma gelsolin in ovarian cancer chemoresistance. Oncogene. 2020, 39, 1600–16. 10.1038/s41388-019-1087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorayappan K. D. P.; Gardner M. L.; Hisey C. L.; Zingarelli R. A.; Smith B. Q.; Lightfoot M. D. S.; et al. A Microfluidic Chip Enables Isolation of Exosomes and Establishment of Their Protein Profiles and Associated Signaling Pathways in Ovarian Cancer. Cancer research. 2019, 79, 3503–13. 10.1158/0008-5472.CAN-18-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdian-shakib A.; Dorostkar R.; Tat M.; Hashemzadeh M. S.; Saidi N.; et al. Differential role of microRNAs in prognosis, diagnosis, and therapy of ovarian cancer. Biomed. Pharmacother. 2016, 84, 592–600. 10.1016/j.biopha.2016.09.087. [DOI] [PubMed] [Google Scholar]
- Kanlikilicer P.; Rashed M. H.; Bayraktar R.; Mitra R.; Ivan C.; Aslan B.; et al. Ubiquitous Release of Exosomal Tumor Suppressor miR-6126 from Ovarian Cancer Cells. Cancer research. 2016, 76, 7194–207. 10.1158/0008-5472.CAN-16-0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Leva G.; Garofalo M.; Croce C. M. MicroRNAs in cancer. Annual Review of Pathology: Mechanisms of Disease. 2014, 9, 287–314. 10.1146/annurev-pathol-012513-104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales R. T.; Ko J. Future of Digital Assays to Resolve Clinical Heterogeneity of Single Extracellular Vesicles. ACS Nano 2022, 16 (8), 11619–45. 10.1021/acsnano.2c04337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R.; Gorai S.; Devi A.; Muthusamy R.; Alexiou A.; Papadakis M. Decoding of exosome heterogeneity for cancer theranostics. Clin Transl Med. 2023, 13, e1288 10.1002/ctm2.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H.; Zhang L.; Zhao Y.; Yang D.; Song F.; et al. Plasma miRNAs as diagnostic and prognostic biomarkers for ovarian cancer. PLoS One. 2013, 8, e77853 10.1371/journal.pone.0077853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X.; Liu S.; Liu Y.; Lin X.; Zheng T.; et al. Circulating serum exosomal aHIF is a novel prognostic predictor for epithelial ovarian cancer. Onco Targets Ther. 2019, 12, 7699–7711. 10.2147/OTT.S220533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell A. A.; Neupane K. R.; McCorkle J. R.; Fu X.; Moonschi F. H.; Caudill E. B.; Kolesar J.; Richards C. I. Cell-Derived Vesicles for in Vitro and in Vivo Targeted Therapeutic Delivery. ACS Omega. 2019, 4, 12657–12664. 10.1021/acsomega.9b01353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra N.; Dutt Arya B.; Jain N.; Yadav P.; Wajid S.; Singh S. P.; Choudhury S. Biophysical Characterization and Drug Delivery Potential of Exosomes from Human Wharton’s Jelly-Derived Mesenchymal Stem Cells. ACS Omega. 2019, 4, 13143–13152. 10.1021/acsomega.9b01180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh S. K.; Khan M. A.; Singh S.; Singh A. P. Extracellular Nanovesicles: From Intercellular Messengers to Efficient Drug Delivery Systems. ACS Omega. 2021, 6, 1773–1779. 10.1021/acsomega.0c05539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanchanapally R.; Khan M. A.; Deshmukh S. K.; Srivastava S. K.; Khushman M.; Singh S.; Singh A. P. Exosomal Formulation Escalates Cellular Uptake of Honokiol Leading to the Enhancement of Its Antitumor Efficacy. ACS Omega. 2020, 5, 23299–23307. 10.1021/acsomega.0c03136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasser T. A.; Adel R.; Badr A.; Teleb M.; Bekhit A. A.; Elkhodairy K. A.; Abdelhamid A. S.; Elzoghby A. O. Combined Cancer Immunotheranostic Nanomedicines: Delivery Technologies and Therapeutic Outcomes. ACS Omega. 2023, 8, 4491–4507. 10.1021/acsomega.2c05986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antimisiaris S. G.; Mourtas S.; Marazioti A. Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery. Pharmaceutics. 2018, 10, 218. 10.3390/pharmaceutics10040218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen K. B.; Gudbergsson J. M.; Skov M. N.; Pilgaard L.; Moos T.; Duroux M. A comprehensive overview of exosomes as drug delivery vehicles – endogenous nanocarriers for targeted cancer therapy. Biochimica et biophysica acta. 2014, 1846, 75–87. 10.1016/j.bbcan.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Gao D.; Jiang L. Exosomes in cancer therapy: a novel experimental strategy. Am. J. Cancer Res. 2018, 8, 2165–75. [PMC free article] [PubMed] [Google Scholar]
- Salehpour A.; Balmagambetova S.; Mussin N.; Kaliyev A.; Rahmanifar F. Mesenchymal stromal/stem cell-derived exosomes and genitourinary cancers: A mini review. Front Cell Dev Biol. 2023, 10, 1115786. 10.3389/fcell.2022.1115786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Z. L.; Li J. F.; Luo H. M.; Liu Y. Y.; Jin Y. Plant extracellular vesicles: A novel bioactive nanoparticle for tumor therapy. Front Pharmacol. 2022, 13, 1006299. 10.3389/fphar.2022.1006299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X. J.; Sun X. Y.; Huang K. M.; Zhang L.; Yang Z. S.; Zou D. D.; Wang B.; Warnock G. L.; Dai L. J.; Luo J. Therapeutic potential of CAR-T cell-derived exosomes: a cell-free modality for targeted cancer therapy. Oncotarget. 2015, 6, 44179–90. 10.18632/oncotarget.6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W.; Lei C.; Liu S.; Cui Y.; Wang C.; Qian K.; Li T.; Shen Y.; Fan X.; Lin F.; Ding M.; Pan M.; Ye X.; Yang Y.; Hu S. CAR exosomes derived from effector CAR-T cells have potent antitumour effects and low toxicity. Nat. Commun. 2019, 10, 4355. 10.1038/s41467-019-12321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.; Zhao J.; Tian F.; Chang J.; Zhang W.; Sun J. λ-DNA- and Aptamer-Mediated Sorting and Analysis of Extracellular Vesicles. J. Am. Chem. Soc. 2019, 141, 3817–21. 10.1021/jacs.9b00007. [DOI] [PubMed] [Google Scholar]
- Chen W. W.; Balaj L.; Liau L. M.; Samuels M. L.; Kotsopoulos S. K.; Maguire C. A.; et al. BEAMing and Droplet Digital PCR Analysis of Mutant IDH1 mRNA in Glioma Patient Serum and Cerebrospinal Fluid Extracellular Vesicles. Mol. Ther Nucleic Acids. 2013, 2, e109 10.1038/mtna.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan B. M.; Bashir R. Nanopore sensors for nucleic acid analysis. Nature nanotechnology. 2011, 6, 615–24. 10.1038/nnano.2011.129. [DOI] [PubMed] [Google Scholar]
- Xing S.; Lu Z.; Huang Q.; Li H.; Wang Y.; Lai Y.; et al. An ultrasensitive hybridization chain reaction-amplified CRISPR-Cas12a aptasensor for extracellular vesicle surface protein quantification. Theranostics. 2020, 10, 10262–73. 10.7150/thno.49047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin L. K.; Son T.; Hong J. S.; Liu A. Q.; Skog J.; Castro C. M.; et al. Plasmonic Sensors for Extracellular Vesicle Analysis: From Scientific Development to Translational Research. ACS Nano 2020, 14, 14528–48. 10.1021/acsnano.0c07581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R.; Gorai S.; Devi A.; Jha S. K.; Rahman M. A.; Alexiou A.; Papadakis M.; et al. Exosome: A megastar of future cancer personalized and precision medicine. Clin Transl. Disc. 2023, 3, e208 10.1002/ctd2.208. [DOI] [Google Scholar]
- Chen C.; Zong S.; Liu Y.; Wang Z.; Zhang Y.; Chen B.; Cui Y.; et al. Profiling of Exosomal Biomarkers for Accurate Cancer Identification: Combining DNA-PAINT with Machine- Learning-Based Classification. Small (Weinheim an der Bergstrasse, Germany). 2019, 15, 1901014 10.1002/smll.201901014. [DOI] [PubMed] [Google Scholar]
- Bowers E. C.; Hassanin A. A. I.; Ramos K. S. In vitro models of exosome biology and toxicology: New frontiers in biomedical research. Toxicol In Vitro. 2020, 64, 104462. 10.1016/j.tiv.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha D. H.; Kim H. K.; Lee J.; Kwon H. H.; Park G. H.; et al. Mesenchymal Stem/Stromal Cell Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells. 2020, 9, 1157. 10.3390/cells9051157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R.; Mukerjee N.; Mukherjee D.; Devi A.; Jha S. K.; Gorai S. Plant-derived exosomes: A new dimension in cancer therapy. Phytother Res. 2023, 10.1002/ptr.7828. [DOI] [PubMed] [Google Scholar]
- Dhar R.; Bhattacharya B.; Mandal D.; Devi A.; Thorat N. D. Exosome-based cancer vaccine: A cutting-edge approach - Correspondence. Int. J. Surg. 2022, 108, 106993. 10.1016/j.ijsu.2022.106993. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.