Abstract
Research on neural interfaces has historically concentrated on development of systems for the brain; however, there is increasing interest in peripheral nerve interfaces (PNIs) that could provide benefit when peripheral nerve function is compromised, such as for amputees. Efforts focus on designing scalable and high-performance sensory and motor peripheral nervous system interfaces. Current PNIs face several design challenges such as undersampling of signals from the thousands of axons, nerve-fiber selectivity, and device–tissue integration. To improve PNIs, several researchers have turned to tissue engineering. Peripheral nerve tissue engineering has focused on designing regeneration scaffolds that mimic normal nerve extracellular matrix composition, provide advanced microarchitecture to stimulate cell migration, and have mechanical properties like the native nerve. By combining PNIs with tissue engineering, the goal is to promote natural axon regeneration into the devices to facilitate close contact with electrodes; in contrast, traditional PNIs rely on insertion or placement of electrodes into or around existing nerves, or do not utilize materials to actively facilitate axon regeneration. This review presents the state-of-the-art of PNIs and nerve tissue engineering, highlights recent approaches to combine neural-interface technology and tissue engineering, and addresses the remaining challenges with foreign-body response.
Keywords: bioelectronic medicine, neural interfaces, neural recording and stimulation, peripheral nerve regeneration, sensory-motor prosthesis
1. Introduction
The desire to improve human understanding of the nervous system, and the means to restore or correct its function in the case of injury or disease, has driven researchers for well over 100 years. As new state-of-the-art technologies are developed, multidisciplinary teams of scientists, engineers, and clinicians partner to adapt their use in the context of neuroscience research and clinical therapies. Currently there exist many neurotechnology translation efforts that have seen clinical and commercial successes, including neuromodulation devices used for management of pain and movement disorders.[1–5]
Although the specific requirements needed for each neurotechnology to effectively sense and/or modulate the neural activity in different tissue targets can vary depending on application; it is essential that all interfaces maintain their functional integrity over time. It is for this reason that efforts to develop an implantable neuro-technology must contend with the challenges associated with the tissue-technology interface. The most common approach is re-engineering the interface to reduce or eliminate adverse effects by reducing size, changing materials, increasing mechanical flexibility, or eliminating tethers/leads with wireless telemetry. Here, we discuss another approach which is to address the challenges of the tissue-technology interface and improve the performance of neurotechnology implants by combining the fields of neural-interface technology with tissue engineering.
In the past several years the recognition of the need for improved peripheral nerve interfaces (PNI) for both existing and emerging applications has been rapidly growing.[6–11] An existing peripheral nervous system (PNS) application that is driving the need for improved PNI technology is to serve amputees, who need scalable and high-performance sensory and motor PNS interfaces to control state-of-the-art prosthetic limbs and to receive high-resolution and multi-modal (e.g., tactile and proprioceptive) feedback from sensors integrated into prosthetic limbs.[12]
For amputees, prosthetic-limb technology has been rapidly advancing. Current prosthetic limbs now have over 20 independent degrees of freedom and are increasingly capable of capturing various forms of sensory information (e.g., pressure, shear, temperature).[13] Despite this remarkable progress in robotic limb technology, advances in neural-interface technology have not kept up at the same pace.[13] To provide fine movement control and to elicit high-resolution sensory percepts to exploit the full capability of state-of-the-art limbs, a comprehensive bidirectional nerve-interface would need a large number of independent motor and sensory channels. However, all existing PNI approaches have one or more of the following challenges that limit their clinical translatability, overall utility, ultimate performance, and operation lifetime: low channel count, low signal-to-noise ratio, low selectivity, high foreign-body response, susceptible to motion artifacts, and incompatibility with possible nerve swelling.
An emerging PNS application for neural-interface technology is to sense and modulate the neural activity of autonomic nerves to improve the function of visceral organs in the case of many different diseases (e.g., respiratory failure, chronic inflammation, rheumatoid arthritis, diabetes, and obesity). The name of this emerging field for PNIs is called bio-electronic medicines.[10,14] Most efforts to develop bioelectronics-medicine-based neurotechnology for use in the clinic currently use a conventional vagal nerve-stimulation interface, which is a large single-channel PNS interface. However, to achieve the full potential benefits and simultaneously avoid the potential adverse side effects of bioelectronic medicines, more advanced PNS interfaces with greater selectivity and higher sensitivity (e.g., higher interface channel counts, density, and spatial range), such as those currently needed for amputees, may enable more precise and reliable control of autonomic function.
This paper reviews peripheral nerve interface technologies, peripheral-nerve-tissue engineering approaches, remaining challenges in the field, and future directions. Specifically, we discuss how the fields of neural interfaces and tissue engineering could merge collaboratively to address the challenges of the tissue-technology interface and maximize the performance of neurotechnology implants. The review begins by discussing prior and current PNI technologies and their unique strengths/weaknesses to meet the needs of amputees and/or bioelectronic medicines. Next, we review prior and current tissue engineering methods used for peripheral nerves, and describe their capabilities and limitations. We then follow those two review sections with a description of how the fields of tissue engineering and neural interfaces could be merged to address some of the remaining challenges in the field.
2. Traditional Peripheral Nerve Interface (PNI) Approaches
Peripheral nerves have an anatomical structure that varies widely throughout the body. Large nerves can have many fascicles (i.e., clusters of axons grouped together and surrounded by a tightly packed cellular layer called the perineurium), while smaller nerves may have only a single fascicle. There are both sensory and motor fibers within a nerve trunk, and their number and spatial distribution also varies widely throughout the body.[15–18] When recording from nerves, the largest signal sources are the nodes of Ranvier, which are spaced differently along each fiber are about 0.2 to 2 mm apart.[19] The result is a 3D cloud of nodes of Ranvier dispersed throughout each nerve. To extensively capture the electrical activity of a nerve, which may have anywhere from hundreds to tens of thousands of fibers depending on the size and location, a neural interface should also be 3D in nature. The influence of the size and location of electrodes inside or around nerves on the nature of signals that are recorded from peripheral nerves is summarized in Figure 1.
Figure 1.
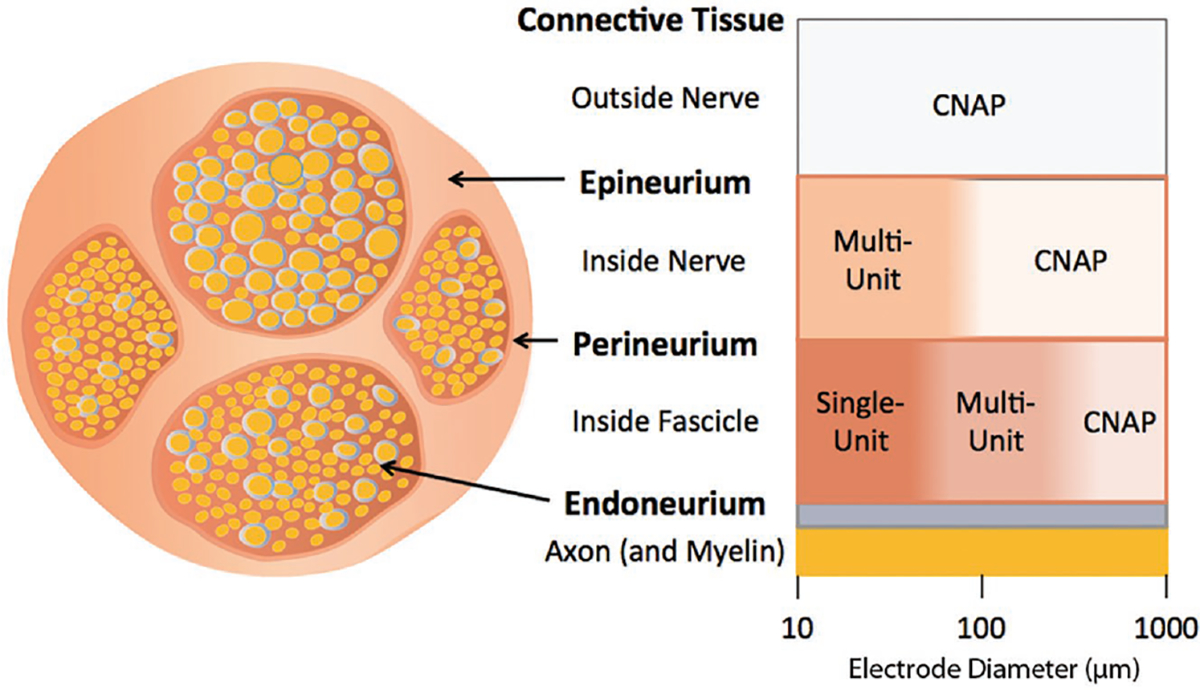
Recorded nerve signal as a function of electrode location around or inside a nerve and electrode size. This diagram shows the relationship between electrode diameter and location of the electrode relative to signal specificity. Single-unit signals are recorded from small-sized electrodes (≈10–100 μm) inside the perineurium, representing action potentials detected from individual axons, typically extracted by spike-sorting. Multiunit signals are recorded from larger-sized (≈100–1000 μm) electrodes, either inside the perineurium or outside the epineurium, representing activity derived from aggregate action-potential spikes of multiple axons that are indistinguishable through spike sorting. Compound nerve action potentials (CNAP) are detectable on the outside of the nerve for any electrode size or inside the nerve for larger electrodes, representing cumulative electric potentials generated in the nerve.
Bioelectrical interfaces to peripheral nerves must contend with the heterogeneity in nerve geometry, fascicular arrangement, and fiber composition. In addition, the close proximity of muscles, tissue movement, and tissue compression can lead to large-amplitude (mV range) interfering signal (noise) sources,[20,21] which can make it difficult to record the small-amplitude (μV range) nerve signals.
To date, several different strategies have been used to interface with peripheral nerves at varying levels of complexity and invasiveness. As with bioelectrical interfaces to the brain, there exists a tradeoff between invasiveness and both signal-to-noise ratio and spatial resolution.
2.1. Nerve Cuffs
An effective early strategy to chronically record[22] and stimulate[23] nerve activity, was to wrap them with a biocompatible dielectric material (e.g., silicone) that had microwire electrodes integrated into the inner surface of the wrap (Figure 2). Microfabrication processes can be used to produce nerve cuffs that integrate thin films of a polymer dielectric with thin-film microelectrodes.[24] These processes can produce smaller and higher resolution nerve cuffs.[25]
Figure 2.
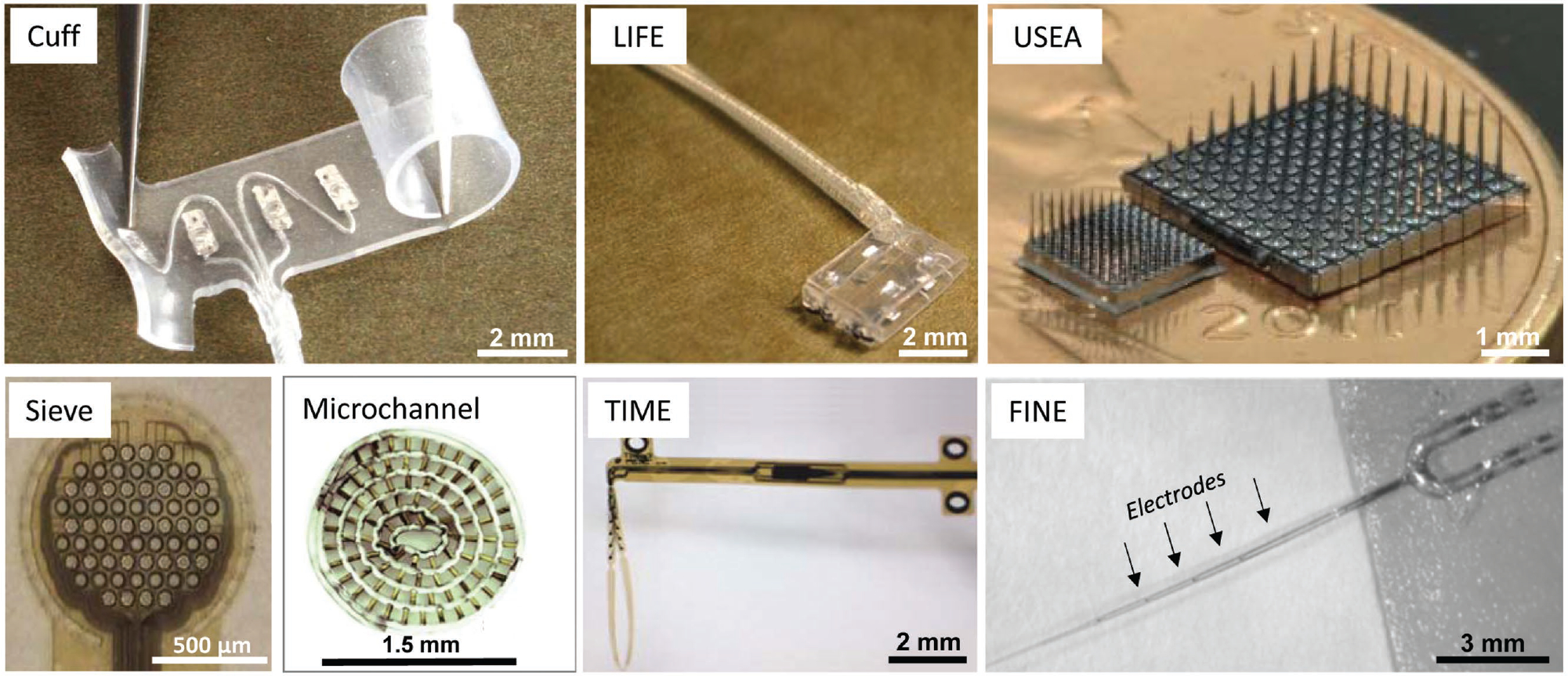
Traditional peripheral nerve interface (PNI) approaches. Several different strategies have been used to interface with peripheral nerves, which can be broadly classified as non-penetrating, penetrating, or regenerative electrodes. Non-penetrating electrodes: Nerve cuff. Reproduced with permission.[26] Copyright 2017, Ardiem Medical. FINE: flat-interface nerve electrode. Reproduced with permission.[27] Copyright 2013, IOP. Penetrating electrodes: LIFE: longitudinal intrafascicular electrode. Reproduced with permission.[28] Copyright 2007, IEEE. TIME: transverse intrafascicular multichannel electrode. Reproduced with permission.[29] Copyright 2011, Wiley. USEA: Utah slanted electrode array. Reproduced with permission.[30] Copyright 2013, IOP. Regenerative electrodes: Sieve. Reproduced with permission.[31] Copyright 2016, IEEE. Microchannel. Reproduced with permission.[32] Copyright 2015, Elsevier. For a more complete depiction of PNI device configurations, see Table II in a comprehensive review by Kim and Romero-Ortega.[33]
The strengths of the nerve cuff are that it is simple, inexpensive, made of relatively soft and biocompatible materials, and foreign body responses can encapsulate it with the nerve, thus stabilizing its relative positioning.[34] However, since nerve-cuff electrodes are positioned around the outside of a nerve, the perineurium and epineurium layers largely attenuate signal amplitude (e.g., ≈5–10 μV). In fact, given the large size and few number of electrode contacts, traditional cuffs do not have fiber-level selectivity and can only sense compound nerve action potentials (CNAPs) and multi-unit activity.[35] Since traditional cuffs are best suited for nerve-interface applications that do not require many channels of independent motor and sensory information, they are not well suited to serve the amputee population.
2.2. FINE: Flat-Interface Nerve Electrode
The FINE–nerve interface is an advanced form of the nerve cuff that can have a higher channel count and has a geometry that causes the nerve to remodel into a flatter non-circular configuration. In the process of flattening out, the fascicles in the nerve spread out and end up being closer to one or more of the densely arranged electrodes (Figure 2).[36] This fact results in a core strength of the FINE–nerve interface, which is improved localization of recorded neural activity and targeted neural stimulation compared with conventional nerve cuffs.[27] Another strength is that the biocompatible materials of the device and relatively conventional manufacturing process has led to rapid demonstrations of chronic viability[37] and quicker clinical translation and regulatory approval than other approaches.[38]
Although the FINE–nerve-interface approach has greater selectivity than nerve cuffs (i.e., can more easily target specific fascicles), its selectivity is still limited because of the large size of the electrodes and the still significant distance between the electrodes and the nerve fibers. This is particularly true for recording spontaneous nerve activity from individual or small groups of fibers, because of the reduction in amplitude as the signal travels through the perineurium and the epineurium. As a result, reliable recordings with FINE–nerve interfaces are still limited to electrically evoked compound action potentials, rather than spontaneous single-unit nerve activity.[39]
2.3. LIFE: Longitudinal Intra-Fascicular Electrode
Initially the LIFE–nerve interface consisted of individual insulated small-diameter (25 μm) platinum microwires with insulation removed from a 0.25 mm long to 1.5 mm long section. The fine microwire is then attached to the back end of a tungsten needle, which is then threaded through a fascicle in a nerve. Studies with human amputee test subjects demonstrated that TF-LIFE–nerve interfaces can provide sufficient sensory feedback information to precisely regulate grip strength, limb position, and the control of hand movements,[40–42] albeit with the use wavelet signal processing.[43]
The strengths of the LIFE–nerve interface include a higher signal-to-noise ratio and a lower stimulation threshold than the cuff and FINE approaches. This is caused by being inside the epineurium and perineurium, where the distance between the LIFE electrodes and the axons is greatly reduced, and the relatively high impedance of the perineurium tends to trap current inside the fascicle and reduces the decay of potential changes inside the fascicle.[44]
However, a challenge for the conventional microwire LIFE is the stiffness mismatch between a solid metal wire (elastic modulus of Pt wire is ≈168 GPa) and nerve tissue (≈10 kPa).[45,46] This mismatch, which results in differential motion between the wire and the tissue, ultimately leads to an adverse fibrotic tissue response, a drift in recorded fiber population, and a significant reduction in signal-to-noise ratio.[47] Subsequent efforts to develop smaller (12 μm diameter) and more flexible LIFE–nerve interfaces (e.g., ≈3 GPa) that have a polymer core (Kevlar) with a thin metal coating, have resulted in a greatly reduced tissue response but have yet to show a sustained improvement in recorded signal-to-noise ratio.[28,48] Alternatively, microfabrication procedures can be used to produce multi-electrode thin-film (TF-LIFE) nerve interfaces[49] that have high flexibility (Figure 2). However, the linear arrangement of electrodes in the LIFE electrode allows sampling of only the small volume of the nerve adjacent to the single line of electrodes.
2.4. TIME: Transverse Intrafascicular Multichannel Electrode
The TIME–nerve interface was developed to achieve good contact with nerve fibers, selectively addresses several fascicles in a nerve with a single implant, and minimizes the mechanical mismatch between the implanted material and nerve tissue (Figure 2).[50] It is essentially the same as the TF-LIFE except that it is implanted across the width of the nerve instead of along its length as is done with the LIFE–nerve interface.[50,51]
The strengths of the TIME–nerve interface include the positioning of small microelectrodes inside the nerve close to the fibers and sometimes inside fascicles, having a relatively high number of independent channels (typically <32), and being manufactured with relatively soft biocompatible materials (e.g., polyimide). The TIME–nerve interface has also been used in human amputees to stimulate the median and ulnar nerves to convey real-time and physiologically appropriate grasping and control information from prosthesis-integrated sensors.[52] Similar to the LIFE electrode, the linear arrangement of electrodes in the TIME interface samples only the small volume of the nerve adjacent to the single line of electrodes. Also, another limitation of the TIME interface is that some electrodes could be positioned in between fascicles by virtue of the implantation technique, which could lead to sub-optimal selectivity in stimulation and recording.
2.5. USEA: Utah Slant Electrode Array
As the field of neuroscience has progressed, there has been a push to increase the number of microelectrodes used to interface with tissue at a cellular level. To meet this demand microfabrication processes have been used to produce dense arrays of microprobes, such as the Utah Electrode Array (UEA).[53,54] The 100-channel UEA, which consists of 1.5-mm-long probes tipped by microelectrodes, has been widely used to successfully interface with cortex[55] to record and/or stimulate the action potentials of individual cells. To interface with nerves, the USEA was developed with rows of microprobes of increasing length (Figure 2).[56] Recent work has increased the USEA microprobe density by a factor of 4[30] from 6.25 electrodes per mm2 (USEA) to 25 electrodes per mm2 (high density-USEA).
The strengths of the USEA include its ability to penetrate the nerve, densely place a large number of microelectrodes inside the nerve at different depths and adjacent to different fibers, record and/or stimulate action potentials with each channel, and do so with a core technology (UEA) that has already received FDA approval for use in humans. These strengths have enabled the USEAs to successful interface with the ulnar and median nerves of human amputees to record single-unit action potentials and decode movement intent, as well as provide sensory feedback from prostheses.[57,58] However, a key limitation of the UEA/USEA technology is its well-known limited reliability.[59] Specifically, it has been shown that the number of channels on the UEA with detectable neural signals falls by ≈60% per year, even in the hands of the most experienced researchers.[60] It may appear from the end-on view of electrode arrangement and their proximity to all of the fibers that the USEA will be able to engage with most of the nerve fibers. However, the 2D arrangement of electrodes in USEAs still significantly under-sample the 3D cloud manifestation of the nodes of Ranvier, and hence fibers of a nerve. Because the USEA has small-sized electrodes that are located not only inside the epineurium, but often inside the perineurium, it can capture larger amplitude compound nerve action potential (CNAP) signals as well as single-unit/multi-unit activity, but without fiber-type targeting or selectivity.
2.6. Sieve–Nerve Interface
The concept of entubulation as a surgical intervention for peripheral nerve repair after some laceration injuries (neurotmesis),[61,62] which is summarized in a later section of this paper, has been leveraged in several attempts in order to develop regenerative electrical interfaces with peripheral nerves.[63] An early example is the sieve electrode,[64] which consists of a dielectric that is perforated by many mechanically drilled small-diameter holes and sutured on the end or between the ends of a cut nerve. Regenerated axons will grow through the holes, including those with an adjacent or surrounding electrode to record neural activity (Figure 2). De Luca et al.,[22] reported the first successful recording of voluntary (without electrical stimulation) nerve activation from mammalian peripheral nerves with a simple cuff-based approach. Subsequently, Edell and co-workers[65] reported the first chronic recording of neural signals using silicon-based micromachined sieve-electrode arrays, which led to a series of further studies investigating its potential in longitudinal implant-studies.[63,66–72] Polyimide-based sieve interfaces were later introduced as an alternative to allow fabrication of the electrodes, conducting leads, and contact pads for external connections as a single flexible microfabricated device.[73,74] Despite these improvements, in vivo recording success of polyimide-sieve electrodes was limited to acquiring compound nerve action potentials in anesthetized or terminal end-point preparations, rather than long-term single-unit signals.[75,76] Furthermore, the research focus shifted to the evaluation of regenerated axonal quality and functional recovery of distal target muscles after allowing sufficient time for reinnervation.[77–80] A challenge common to all sieve-electrode approaches is that if the holes in a sieve are too small, axons will either not regenerate through them or suffer from axonopathy (i.e., insufficient room for them to achieve maturation with myelin regrowth and an increase in diameter). However, as the diameter of sieve holes is increased, a critical diameter is reached where the number of fibers regenerating through the holes will go from zero to many, which makes it very difficult to distinguish recordings. Also, the planar arrangement of electrodes in the sieve can permit limited sampling of the 3D volume of nodes of Ranvier in nerves. A different engineering design is needed that does not impose so many constraints on the regenerating axons, yet provides a robust and scalable means for interfacing with the regenerated nerve fibers.
2.7. Microchannel–Nerve Interface
The microchannel interface builds upon the sieve-interface concept by elongating the holes into long tubes within which axons can regrow (Figure 2). The first attempt demonstrated the regeneration of bullfrog sciatic nerves through porous Teflon implants embedded with 25-μm-diameter hollow gold cylinders.[81] Although subsequent attempts involved the formation of multiple channels made of dielectric lined with passive conducting Au-Pt metals[82] or MOSFET buffer transistors,[83] none of these early designs were reported to successfully obtain in vivo recordings of neural activity. Recent microchannel-interface designs have explored the idea of restricting the action current leaving the nodes of Ranvier and forcing it to flow parallel to the axon and longitudinally within the confines of the channel.[84–88] Doing so amplifies extracellular recordings and reduces the charge-injection required for selective stimulation. Recent microchannel interfaces have been constructed from PDMS, which is more elastic (≈1.5 MPa) than silicon (≈200 GPa) or polyimide (≈8 GPa). Histological assessment of tissue regrown through PDMS microchannels has shown successful regeneration of sciatic-nerve axons through these microchannel-based interfaces. Single-unit and multi-unit neural recordings have also been acquired from both acute and chronic implants in rats while they were anesthetized[32] and ambulatory.[89–91]
3. Peripheral Nerve Tissue Engineering Approaches
Advanced neurotechnology applications (e.g., upper limb amputees) require a new approach that overcome the limitations of prior technologies described above. Specifically, required is an interface that provides a scalable channel count; samples over the entire volume described by the diameter of the nerve and the distance between nodes of Ranvier; captures high amplitude CNAP, multi-unit, and single-unit signals; minimizes the negative impacts of tissue movement; mitigates any adverse tissue response; and ideally provides fiber-type targeting and selectivity. We believe that by combining tissue engineering methods with neural-interface technology it is possible to develop a novel tissue-engineered PNI that can meet the challenging demands of amputees seeking to control state-of-the-art prosthetic limbs. A key step towards achieving that goal is to understand the relevant prior work and research trajectories within the field of neural-tissue engineering.
Next-generation PNIs will benefit from strategies developed for peripheral nerve regeneration. To improve the physical and electrical connection between peripheral nerves and electrodes, ideally axons should grow along and near the electrodes. Peripheral nerve tissue engineering approaches and biomimetic strategies may be beneficial for designing a PNI. Peripheral nerve tissue engineering strategies have been explored as alternative solutions to autologous nerve grafts in long-gap nerve damage. The peripheral nervous system has some capacity to regenerate and recover the function in minor nerve damage (e.g., neurapraxia or axonotmesis) through the contributions of myelinating Schwann cells, growth factors, and the biomolecules of the extracellular matrix (ECM).[92] In neurapraxia (temporary blockage of nerve conduction) and axonotmesis (crush injury) no Wallerian degeneration occurs and recovery is typically excellent.[93] In neurotmesis (nerve transection) Wallerian degeneration occurs and recovery is poor without surgical intervention, particularly with longer nerve gap lengths.[93,94] The critical nerve-gap length in the common rat sciatic-nerve model is widely considered to be around 1 cm, and in humans for small diameter nerves is considered to be ≈3 cm.[95,96]
The regenerative process of a transected nerve through a hollow conduit is shown in Figure 3A. Initially, fibrinogen and other blood proteins infiltrate the nerve gap creating an environment rich in proteins and neurotropic factors. Fibrinogen in the presence of thrombin forms fibrin creating a matrix bridging the distal and proximal nerve stumps. Next, pro-regenerative Schwann cells infiltrate the nerve gap, secrete neurotrophic growth factors, and form bands of Büngner. Axons then extend along the fibrin cables and last, differentiated Schwann cells re-myelinate axons. Figure 4 illustrates various tissue engineering strategies, both in research laboratories and in the clinic, to repair and restore the function of damaged peripheral nerves. Various strategies for peripheral nerve repair have been developed, some of which are currently approved for clinical use, while others are being researched as viable future strategies. Strategies being researched in the lab include developing advanced microarchitectures such as aligned filaments, porous sponges, and micro-channels. The use of controlled release of growth factors or embedding supportive cells, such as Schwann cells or stem cells is another strategy being researched. Finally, research is being conducted on mimicking the native ECM found within healthy or regenerating nerves. Current strategies used in the clinic include the current gold standard: autologous nerve grafts where healthy sensory nerves are harvested from elsewhere and replaced at the site of injury. FDA-approved devices include many hollow nerve-guidance conduits and decellularized nerves such as the Avance Nerve Graft developed by AxoGen, Inc.[97,98]
Figure 3.
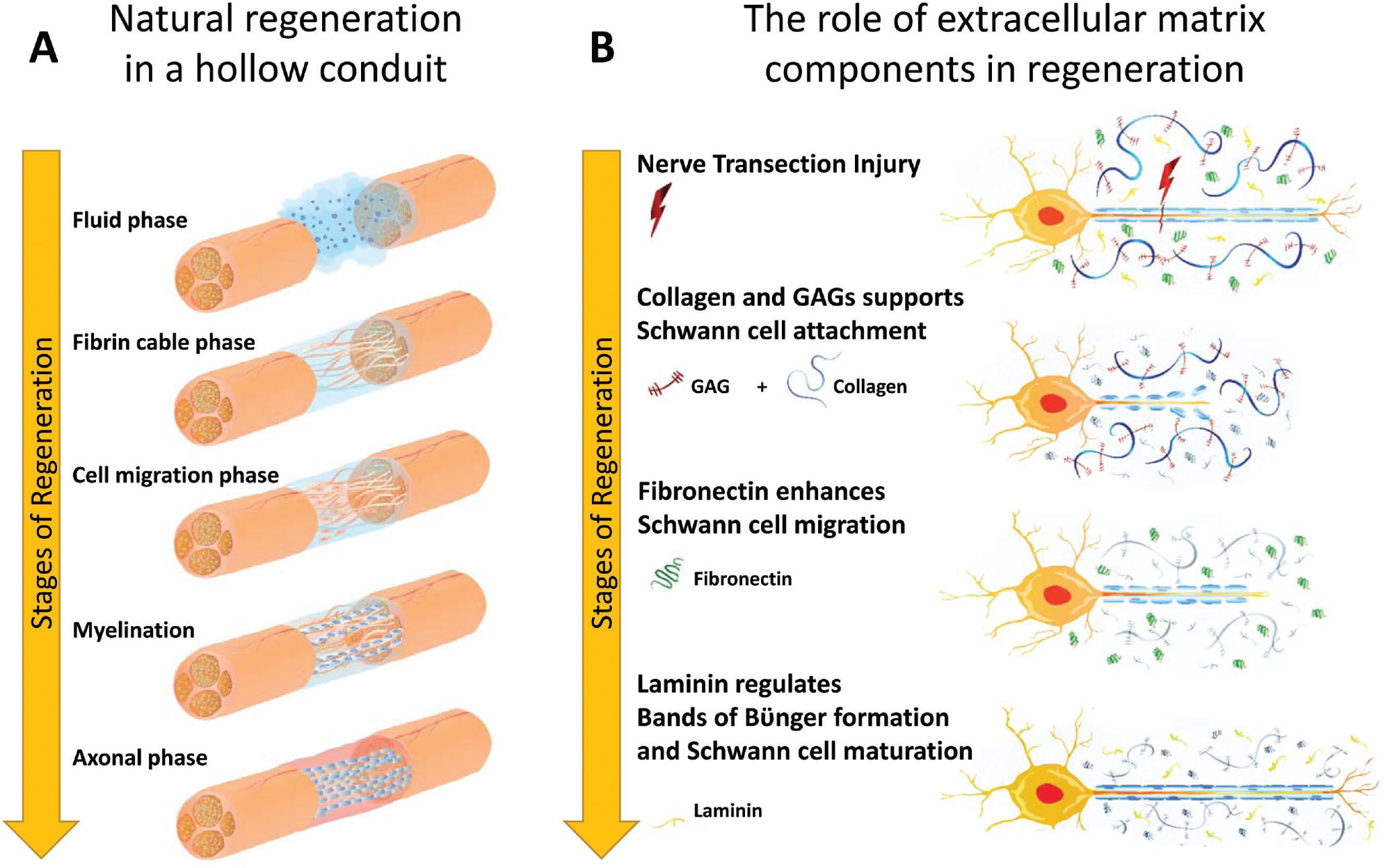
Peripheral nerve regeneration. A) Natural regeneration in a hollow conduit undergoes 5 phases: 1) Fluid phase: fibrinogen and other blood proteins infiltrate the nerve gap and the area become rich in protein and neurotropic factors. 2) Fibrin cable phase: fibrinogen in the presence of thrombin forms fibrin, which creates a matrix bridging the distal and proximal stumps. 3) Cell migration phase: Pro-regenerative Schwann cells infiltrate the gap and secrete trophic factors and form bands of Büngner. 4) Axonal phase: Axons extend along fibrin cables. 5) Myelination phase: Schwann cells re-myelinate axons. B) The role of each extracellular matrix (ECM) component in regeneration. Following nerve transection injury, glycosaminoglycans form a hydrogel network to support cell migration, while Schwann cells attach to the ECM network. Next, fibronectin enhances Schwann cell migration into the nerve. Finally, laminin regulates bands of Büngner formation and Schwann cell maturation, which guides axonal regeneration.
Figure 4.
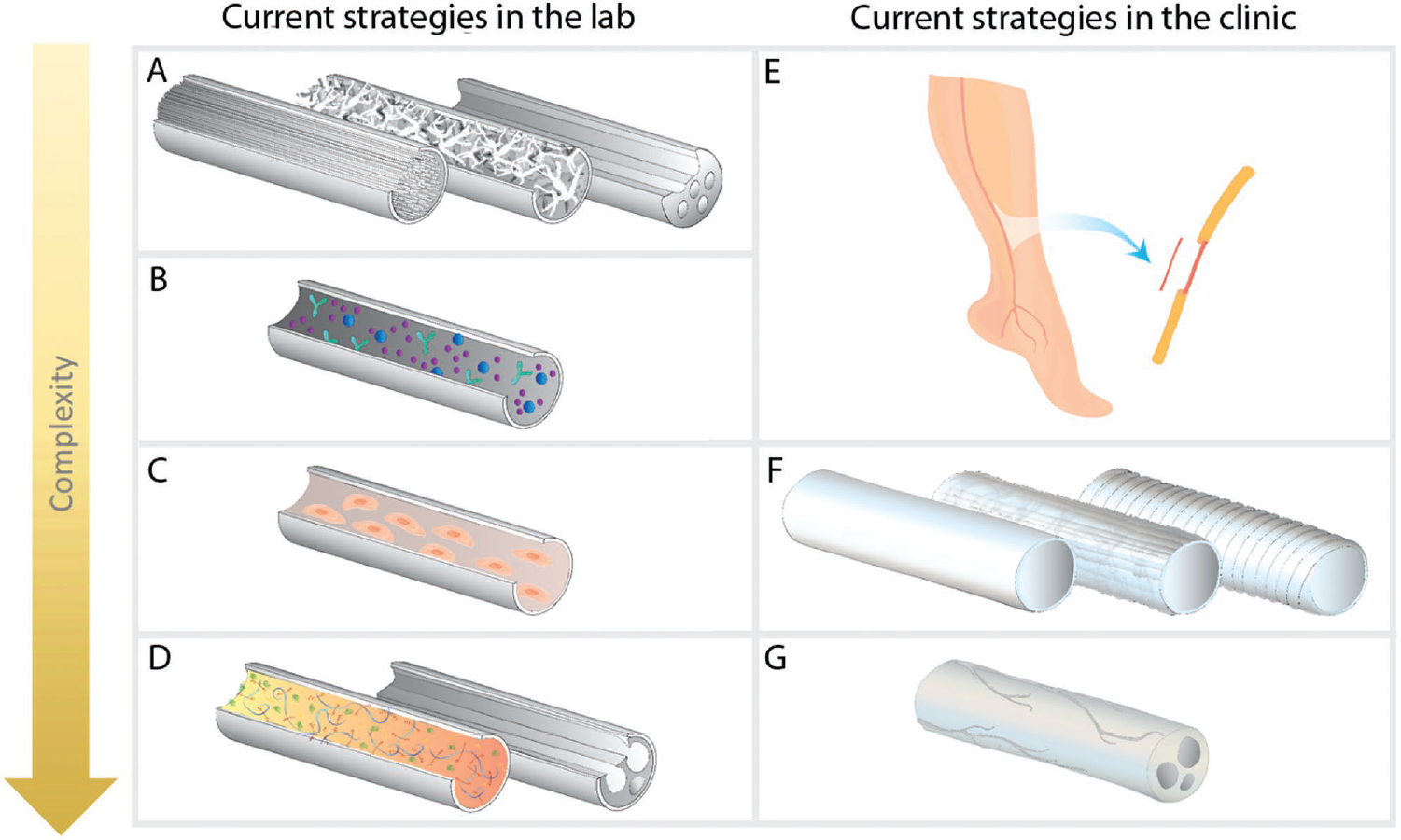
Tissue engineering approaches to peripheral nerve regeneration. Current approaches being researched include A) Developing advanced microarchitecture such as (left to right) aligned filaments,[99] porous sponges,[100] and micro-channels[101] in 3D scaffolds. B) Controlled release of neural growth factors to enhance regeneration.[102] C) Embedding supportive cells, such as Schwann cells[103] or stem cells within 3D scaffolds.[104,105] D) Mimicking natural extracellular matrix (ECM) of the peripheral nerves. To mimic the ECM, it is possible either to combine the components of the ECM[106] or decellularize the natural peripheral nerve tissue.[107] Combinations of above approaches are also being studied to utilize the advantages of various techniques. Current FDA approved techniques and approaches in the clinic include E) Autologous nerve grafts which are the current clinical gold standard for peripheral nerve regeneration. F) Hollow conduits have been translated to clinical product and approved by the FDA. Hollow conduits used in the clinic are composed of either natural (e.g., collagen I) or synthetic (e.g., polyglycolic acid) materials. These products can be either biodegradable or nondegradable. G) Decellularized nerve allografts (AxoGen, Inc.) were more recently approved for use in the clinic.
3.1. Hollow Nerve-Guidance Conduits
Hollow nerve-guidance conduits (NGCs) are the most common off-the-shelf constructs used in the clinic for peripheral nerve repair.[108] NGCs provide simple mechanical support and guidance for regenerating axons. FDA-approved conduits are fabricated from various materials including collagen (e.g., NeuroMend, NeuroMatrix, Neuroflex, NeuraGen, NeuraWrap), porcine small intestine submucosa (SIS) (e.g., AxoGuard Nerve Connector/Protector), polyglycolic acid (e.g., Neurotube), polylactic acid co-polycaprolactone (e.g., Neurolac), and polyvinyl alcohol.[108,109] The process for regeneration through a hollow conduit is illustrated in Figure 3A. In the context of PNIs, NGCs can serve as a frame for the electrodes and host the nerve stump, while providing the structural guidance cues for axons to regenerate.
Recent approaches to peripheral nerve tissue engineering involve fabrication of bioengineered 3D scaffolds that closely mimic natural peripheral nerve tissue. Current strategies include mimicking the microarchitecture of the native nerve tissue or the composition and physical properties of the ECM, or a combination of both strategies.[110]
3.2. Mimicking Microarchitecture
A scaffold’s microarchitecture can provide excellent physical guidance cues for axonal extension (Figure 5). Various microarchitectures have been designed and studied for peripheral nerve regeneration, such as micro-channels (mimicking the native basal lamina of peripheral nerve),[111] aligned fibers,[112] longitudinally aligned porous materials,[113] and multichannel NGCs (mimicking nerve fascicles).[101]
Figure 5.
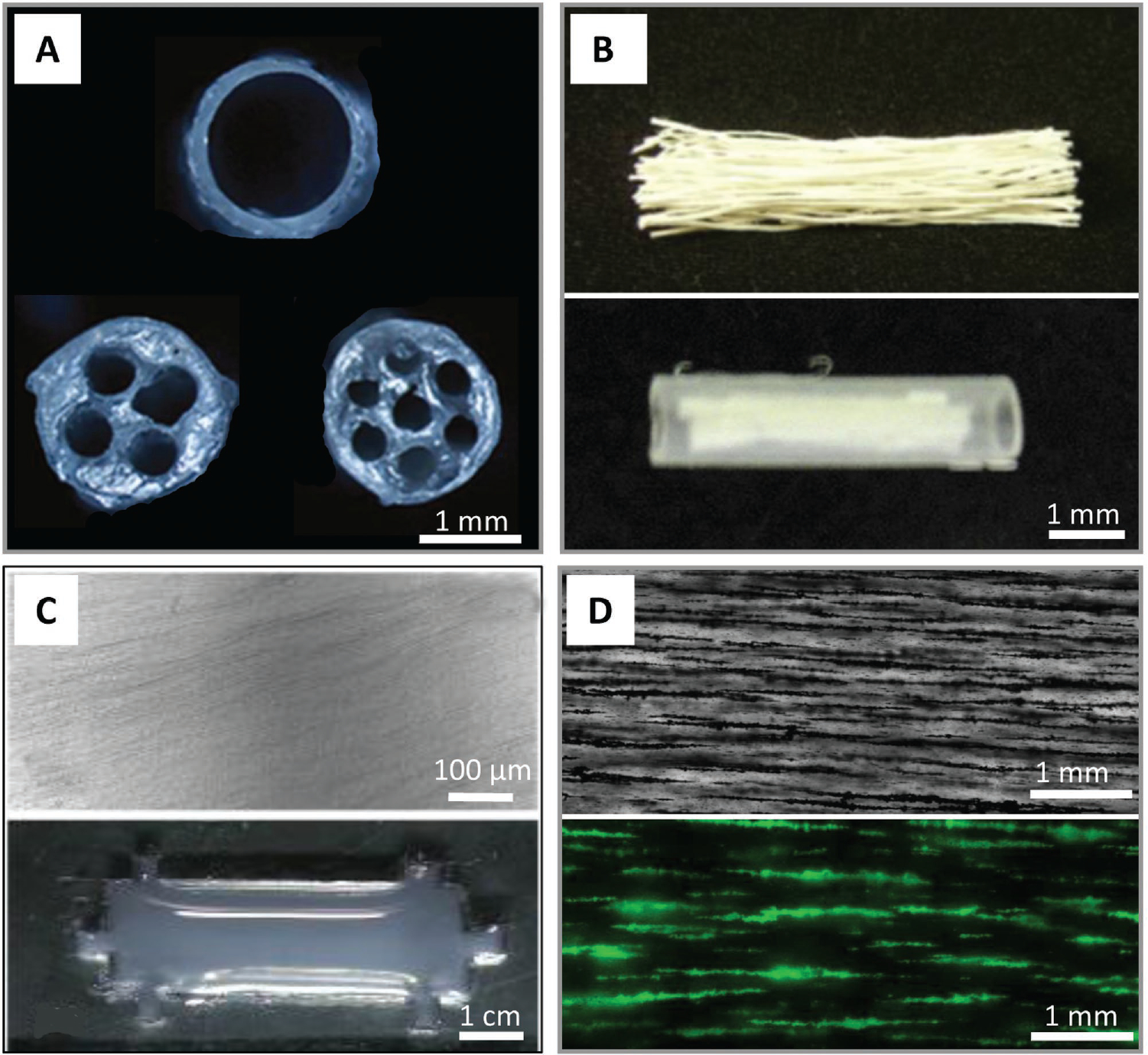
Tissue-engineered peripheral nerve scaffolds/conduits with aligned architectures. A) Multichannel nerve guidance collagen conduits with 1, 4, and 7 channels, respectively. Reproduced with permission.[101] Copyright 2010, Elsevier. B) Linear ordered collagen scaffold fibers within a silicon tube. Reproduced with permission.[112] Copyright 2011, Elsevier. C) Collagen type I hydrogels with aligned collagen fibrils (top); designed with six lateral indentations to anchor contracting collagen fibrils (bottom). Reproduced with permission.[114] Copyright 2009, Elsevier. D) Templated hyaluronic acidbased hydrogels with magnetically aligned microparticles before removal (top). Hydrogel with microchannels following microparticle removal after alignment; channels are visualized by backfilling with dextran-FITC fluorescent dye (bottom).
The earliest architectures were the previously mentioned multichannel NGCs.[101] In this study, the authors prepared collagen conduits with 1, 2, 4, or 7 channels and used the commercially available NeuraGen conduit and an autologous nerve graft (autograft) as controls (Figure 5A). A 1-cm-long rat sciatic nerve gap was used as the model, over a four-month-long period. Nearly every rat saw myelinated axons midway through the graft after the four-month-long period. However, regeneration was quantitatively analyzed via compound muscle action potential recordings, and the results trended toward lower amplitude with decreased channel count.[101] There are likely two reasons that decreasing the number of channels within a nerve conduit resulted in less regeneration. First, the higher number of channels, the smaller the total cross-sectional area into which axons can regenerate, in addition, although this system does provide some structure, these architectural features are still relatively large and thus do not provide cell and subcellular-level benefits that a system having microarchitecture would.
Though the study is primarily focused on in vitro characterization, Ribeiro-Resende et al.[114] attempted three different methods for inducing the formation of bands of Büngner: aligned extracellular matrices (oriented collagen) (Figure 5C), polarizing differentiation/growth factors (cocktail of three factors: nerve growth factor, neuregulin, and transforming growth factor beta 1), and microstructured biomaterials (polycaprolactone filaments).[114] The optimal system for in vitro Schwann cell alignment was determined to be a combination of oriented collagen plus the three differentiation factors.
Some attempts to mimic the native microarchitecture in a nerve scaffold include Hu et al.’s work on a collagen-chitosan scaffold containing microchannels resembling those of native nerve basal lamina.[111] A 15-mm-long rat sciatic-nerve defect over 4 and 12 weeks was used as the regeneration model, with the autograft, randomly aligned scaffolds, and empty silicone conduits used as controls. After only 4 weeks, all rats implanted with longitudinally aligned scaffolds showed regeneration over the gap, comparable to the positive control autograft. None of the rats with empty conduits displayed full regeneration across the gap, and only 2 out of 9 rats with randomly aligned scaffolds showed regeneration. Similar results were obtained for rats with longitudinally aligned scaffolds, exhibiting functional recovery equivalent to that of the autograft controls.
3.3. Mimicking Nerve Extracellular Matrix
Another regenerative strategy that may benefit regenerative PNIs is ECM-based scaffolds. These scaffolds are either fabricated by combining natural ECM components to form a hydrogel[106] or developed from decellularized nerve tissue.[107] Peripheral nerve ECM includes two main categories of biomolecules: proteoglycans (e.g., glycosaminoglycans) and fibrous proteins (e.g., collagen I, collagen IV, elastin, fibronectin and laminin),[106] which have important roles to effectively guide and enhance regeneration, Figure 3B.
Glycosaminoglycans are hydrophilic polysaccharide chains that can form hydrogels even at low concentrations. Hyaluronic acid is a nonsulfated glycosaminoglycan found in nearly all animal tissues. During regeneration, glycosaminoglycans form a hydrogel network to support cell migration. Early studies on the application of hyaluronic acid for peripheral nerve repair showed that hyaluronic acid resulted in increased conduction velocity 4 weeks post-operation and resulted in higher axon counts in rat sciatic-nerve-injury model.[115]
Collagen I is the most abundant collagen type in nerve tissue and was the earliest studied ECM component to improve nerve regeneration.[116,117] Collagen provides structural guidance during regeneration.[118] With a rat sciatic nerve model, Chamberlain et al. found 6-weeks post-operation a significant increase in the number of axons in the middle of 10-mm-long collagen-glycosaminoglycan scaffolds relative to saline-filled controls of the same length.[119] Because of collagen’s structural role, addition of native microarchitectural features to collagen could improve cell binding and alignment. Ceballos et al. used a magnetic field to align collagen fibers and showed that peripheral nerve regeneration in mice implanted with aligned collagen grafts was significantly higher than for unaligned collagen grafts, after 60 days post-operation.[120]
Laminin is the primary component of basal lamina in nerve tissue. The basal lamina surrounds Schwann cells and forms myelin. Laminin’s adhesive properties can encourage Schwann cell migration during regeneration.[121] During Wallerian degeneration, the basal lamina remains largely intact and interacts with Schwann cells through adhesion proteins, namely integrins α1, α3, α6, and α7. The Schwann cells use the remaining intact basal lamina to longitudinally align and form bands of Büngner that axons use for structural guidance during regeneration.[114] In tissue-engineered ECM scaffolds, laminin is generally combined with other components such as collagen and fibronectin since laminin cannot form a gel on its own. Bailey et al. illustrated that a laminin-filled tube increased the number of Schwann cells migrating into a 5-mm-long gap in a rat sciatic nerve.[122]
Fibronectin forms a fibrillar matrix similar to collagens and mediates cell-binding via integrin α5. Fibronectin enhances Schwann cell migration into the nerve gap following injury by forming a fibrillar matrix that mediates cell-binding. Similar to laminin, fibronectin is generally combined with other components in tissue-engineered ECM scaffolds to improve regeneration during the repair process.[123,124]
3.4. Replicating Mechanical Properties
It is well established in tissue engineering that the mechanical properties of a scaffold are immensely important. Cell behavior such as cell lineage can be significantly modified simply by changing the mechanical properties of the substrate.[125,126] An ideal tissue-engineered nerve scaffold will have mechanical properties similar to that of unmodified nerve. Adult-rat sciatic nerve has been shown to have a Young’s modulus on the order of 580 kPa and an ultimate stress of about 2720 kPa:[127] whereas, another study found that human ulnar and median nerve had Young’s moduli of 12 and 19 kPa, respectively.[45] However, when designing a scaffold for use in a regenerative peripheral nerve interface, the scaffold must be stiff enough to support electrodes during regeneration while not being so stiff as to impede regeneration or the ability to be sutured. Otherwise, an outer conduit that can be sutured may need to be used.
There are not many in vivo studies to define the effect of mechanical properties on the regenerative capacity of neural scaffolds. However, many in vitro studies have been conducted to investigate the changes in cell behavior in different mechanical environments. It remains a challenge to find a compromise between mechanical properties, chemical composition, and architectural features.
Early in vitro experiments generally varied mechanical properties of a hydrogel by changing the hydrogels concentration. Willits and Skornia grew dorsal root ganglia from embryonic chicks in collagen I, ranging in concentration of 0.4 to 2 mg mL−1, for up to 4 days and then quantified neurite extension.[128] Mechanical stiffness was determined via rheology and the range of gel concentrations resulted in storage moduli between 2.0 to 16.9 Pa at 1 rad s−1. Neurite extension was found to be longest at concentrations of approximately 0.6 to 1.0 mg mL−1 of collagen.[128] In these types of studies, modulating the mechanical properties cannot account for other factors, such as interfiber spacing and binding-site availability, which also change based on gel concentration.
Gu et al. developed multiple polyacrylamide gel substrates with matching biochemical properties; however, Young’s moduli ranged from 4.42 to 12.04 kPa, which are lower than the stiffness of native nerve.[129] Schwann cell elongation, adherence, viability, and proliferation were all determined to be optimal at an elastic modulus of 7.45 kPa. Schwann cells cultured on the least stiff gels (4.42 kPa) were found to bunch together, while cells cultured on the other substrates were found to have increased motility. Cells cultured on 7.45 kPa polyacrylamide traveled the furthest and had the highest velocity over a 100-minute-long period.
Another in vitro study has compared culturing Schwann cells and embryonic dorsal root ganglia on substrates with the same biochemical cues but differing stiffness by modifying polyacrylamide concentration.[130] Polyacrylamide gels were generated with elastic moduli of 1, 10, and 20 kPa. This range of stiffness represents the range Schwann cells will experience from development to full maturity. The number of lamellipodia sprouting from Schwann cells was quantified and was found that Schwann cells on weaker gels had more lamellipodia. This indicates that Schwann cells had a low binding affinity to the softer substrates. Schwann cell motility was measured on the various gels over a 6-hour-long period. The least stiff gels resulted in increased distance travelled and higher velocity.[130] As discussed previously, Schwann cell migration is immensely important in regenerating peripheral nerve. This indicates that a softer gel (1–10 kPa) may be able to aid nerve regeneration by increasing Schwann cell motility. However, the two stiffer gels (10 and 20 kPa) resulted in increased length of neurite extensions from dorsal root ganglia and increased linearity of neurites.[130] The range of stiffness used in this study (1, 10, and 20 kPa) was meant to reflect early, intermediate, and late stages of peripheral nerve development. This could account for the differences seen in neurite outgrowth of dorsal root ganglia.
Although measuring Young’s Moduli (tensile or compressive) is commonly used to describe the mechanical properties of biomaterials, there are other considerations to take into account. For example, hydrogel materials display viscoelastic qualities and behave differently than a typical elastic material. This means the viscoelastic properties of a material should also be considered when designing a tissue-engineered scaffold. The primary component in a hydrogel is water, which gives these materials a strong viscous component and behavior. In addition, most tissue, including nerve, is heterogeneous and displays anisotropic behavior.
Continuous cross-talk occurs between a cell sensing the local mechanical properties of its environment and the biochemical cues that co-regulate cell behavior. Integrins act as mechanochemical transducers by converting the mechanical forces near the cell into reactive behavior of the cell.[131] For nerve regeneration, this is highly relevant during growth-cone formation. During embryogenesis or regeneration following nerve injury, a growth cone forms at the end of the axon as it seeks its synaptic target. These growth cones are formed by alignment of actin microfilaments within the axon and are induced by various mechanical and chemical interactions of integrins.[132] It is shown that upregulation in the expression of those genes corresponding to some cell behaviors, such as growth and differentiation, are influenced by mechanical rigidity sensed by the cells. The mRNA expression of critical growth factors such as growth differentiation factors, ciliary neurotrophic factor, and brain-derived neurotrophic factor significantly upregulated in Schwann cells cultured on 7.45 kPa polyacrylamide compared to other polyacrylamide gels of different stiffness (4.42, 9.10, and 10.04 kPa).[129]
There are many considerations for mechanical properties of peripheral nerve interfaces generally. Many PNIs such as the Utah Slant Electrode Array are significantly stiffer than the surrounding tissue, which can result in damage to tissue caused by laceration during device insertion.[33] In implantable brain electrodes this has been cause for significant concern.[133] Common electrodes are 6 to 7 orders of magnitude stiffer than brain tissue, which can result in shear applied to the surrounding tissue and significant damage.[134] Because of these issues, many groups have moved to using more flexible electrode materials such as polyimide and parylene-C;[134] however, these materials can be difficult to implant into neural tissue because the flexibility of these materials makes them difficult to insert into nerve tissue.[134]
3.5. Integrated Strategies
Recent studies suggest that combinations of these strategies will be closer to replicating the natural nerve microenvironment. For example, regeneration in hydrogel-containing conduits resulted in a more mature phenotype, as evidenced by an increase in axon myelination and overall increased nerve cross-sectional area.[124] Rather than using a generic hydrogel, a combination regenerative strategy, which uses primary ECM components along with architectural features that model native nerve, could benefit regenerative PNIs. Native peripheral nerve tissue is a combination of biomolecules, architecture, and cells. In the development of next-generation regenerative PNIs, researchers should consider replicating features of native nerve (e.g., creating a functional biomimicking scaffold mechanically robust enough to hold electrodes in place and biologically functional enough to improve natural axonal regeneration).
4. Combining Tissue Engineering with Peripheral Nerve Interfaces
The development of neural interfaces, which are capable of reliably capturing enough motor information and conveying enough sensory-feedback information to enable intuitive control and full limb embodiment by users of state-of-the-art prosthetic limbs (i.e., providing high-speed, high-resolution, low-latency, and low-error limb interaction), is an unmet open challenge in the neural-interface community. A fundamental limit on the number of independent channels of sensory and motor information is related to the size and number of electrodes. The use of smaller electrodes can enable higher signal-to-noise ratio interaction with fewer nearby cells, but then more electrodes are needed to comprehensively engage with the fibers in a nerve.
In nerves, key targets are the nodes of Ranvier, which are the focal points for transmembrane currents in myelinated fibers. Since the locations of nodes along different fibers are independent of one another, the nodes in a nerve are distributed randomly within a volume equal to the cross-sectional area of the nerve times the average distance between nodes. Thus, the nodes are distributed randomly as a 3D cloud in a cylindrical nerve model. Scalable electrical approaches to thoroughly interface with the nerves must have small electrodes arranged in relatively dense 3D configurations. Although microfabrication processes can be used to produce suitably small electrodes in dense 2D configurations, producing dense 3D configurations and locating them inside the nerve are major challenges to overcome.
The fields of implantable PNIs, tissue engineering, and nerve regeneration are individually well-established and active fields. However, we believe that by combining fields it will be possible to develop PNI approaches that can address the problems limiting the utility and long-term performance of nerve interfaces (Figure 6). Advanced polymer-based multielectrode neural-interface technology has been proven through chronic use in humans as retinal-prosthetic interface,[135] and increasingly used in clinical studies (e.g., TIME and FINE interfaces). In the following sections, we will review existing PNI technologies based on combinations of these two approaches.
Figure 6.
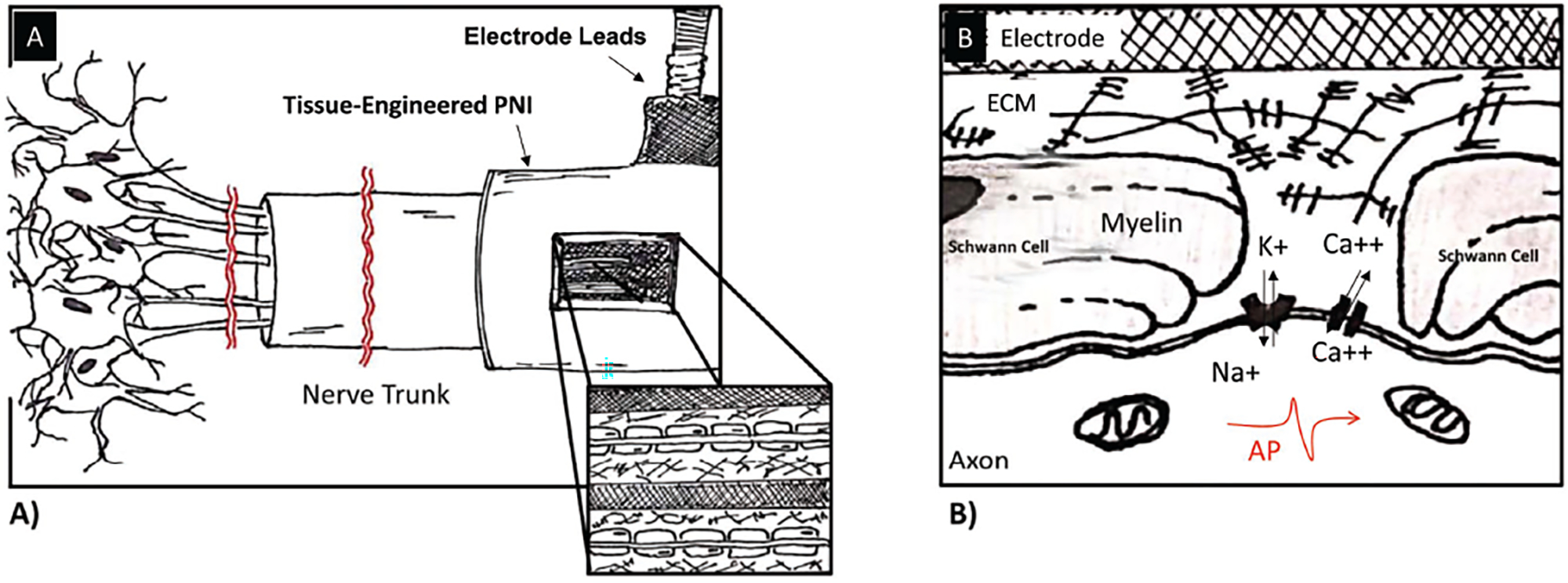
Interaction of tissue-engineered peripheral nerve interface with axons. A) Combining the fields of tissue engineering and neural interfacing will make it possible to develop PNI approaches that can allow for better interactions between axons and electrodes by promoting regeneration of axons into a scaffold that supports the electrodes, which are ideally spaced throughout the entire volume of the device. This distributed configuration of the electrodes will allow for improved interfacing of axons and electrodes and should improve channel count and signal. B) Interactions of axons and electrodes at the cellular level are highly dependent on spacing between nodes of Ranvier and electrodes. Natural ECM will result in functional myelinated axons.
4.1. REMI: Regenerative Multielectrode Interface
The REMI–nerve interface was designed to offer an open-space for axonal regeneration (i.e., less constrictive environment) (Figure 7) in contrast to traditional PNIs such as the sieve–nerve interface.[136] It consists of an array of microwires arranged into a bed-of-nails configuration,[137] but, unlike the USEA, it is not inserted into the nerve. Rather, the REMI–nerve interface is integrated into a hollow polyurethane tube, which is later filled with collagen and then sutured to the ends of a transected peripheral nerve to allow regrowth through the tube. A strength of the REMI includes its ability to record single-unit action potentials (in addition to multi-unit CNAP) because of the small size of the electrodes that are located not only inside the epineurium, but often inside the perineurium.[138] Limitations of the REMI approach include: the use of rigid microwire arrays that result in large mechanical-stiffness mismatch; the use of single-electrode probes fabricated in an array by hand, which limits scalability and reliability of manufacturing compared to batch-microfabricated arrays; the 2D arrangement of REMI electrodes, like the USEA, that ultimately samples only a small fraction of the repeating 3D distribution of nodes of Ranvier; and the use of a simple non-biodegradable conduit, instead of tissue-engineered bioscaffold approaches, which limits robust tissue regeneration and accuracy in tissue targeting.
Figure 7.
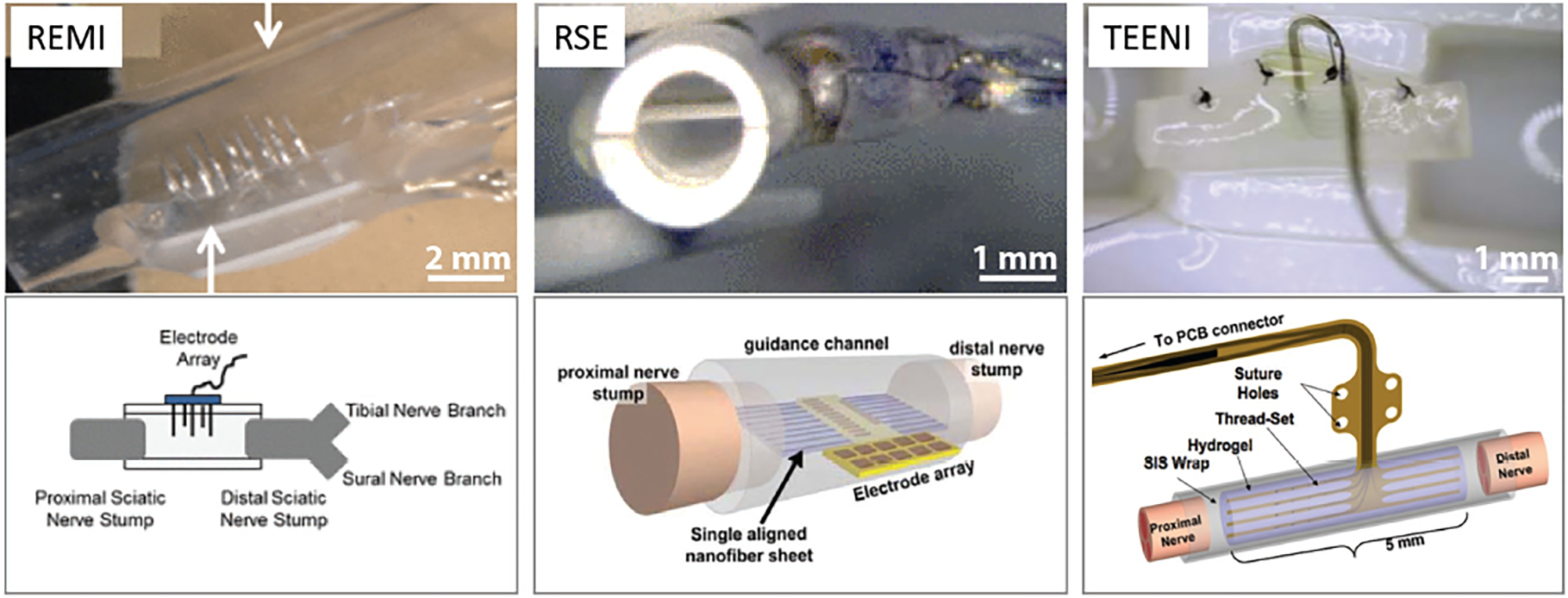
Regenerative peripheral nerve interface approaches. With these designs, the nerve is transected and the device is sutured to the nerve stump; tissue-engineered constructs aid with regeneration into the device. The top images show actual images of the devices and the bottom images are representative schematics of the interfaces. REMI: Regenerative multielectrode interface. Reproduced with permission.[136] Copyright 2012, IEEE. RSE: Regenerative scaffold electrode. Reproduced with permission.[139] Copyright 2013, IEEE. TEENI: Tissue-engineered electronic nerve interface. Reproduced with permission.[140] Copyright 2016, IEEE.
4.2. RPNI: Regenerative Peripheral Nerve Interface
This nerve-interface approach is inspired by the success of the targeted muscle reinnervation (TMR) surgical technique, which eliminates the occurrence of painful neuromas arising from limb amputation.[141–143] Residual nerves from the amputated-limb stump are reattached to alternative muscles in the vicinity (usually in the chest or upper arm) that are surgically denervated. The nerves grow into and reinnervate these muscles and provide physiologically appropriate EMG signals. By doing so, the muscles act as biological amplifiers of motor commands from nerves. The signals, which are large enough to be recorded by electrodes on the surface of the skin, have been used to control robotic prostheses.[141–143]
The RPNI approach differs in two important ways: first, instead of reattaching an entire nerve to a denervated muscle, the fascicles are separated and attached to different denervated muscle targets; and second, the denervated muscle targets are neurotized and free autologous muscle grafts wrapped within a decellularized porcine small intestine submucosa (SIS) tissue layer. The thin SIS layer is either embedded with metal fine wire electrodes or electrochemically plated with poly3,4-ethylenedioxythiophene (PEDOT) for electrical conductivity.[144] After a few months, axons of the severed nerves sprouted and formed new neuromuscular junctions, which were determined to remain viable and functional over six to twelve months.[145,146] Benefits of this approach include the large amplitude of the EMG signals, which makes recording motor-intent signals easier,[147,148] and the use of multiple muscle-graft targets makes it easier to acquire several independent channels of motor-control information. Despite this advancement, the RPNI approach has limitations: the signals required for fine movement might be difficult to acquire from EMG signals and it will be challenging to consistently provide sensory percepts (e.g., tactile) that are evoked by cutaneous receptors not typically found in muscles.
4.3. RSE: Regenerative Scaffold Electrode
An alternative regenerative nerve interface uses a sheet of aligned polymer (PAN-MA) nanofibers to provide a surface that promotes nerve regeneration along its surface which is also placed with a SU-8 substrate based microelectrode array (Figure 7).[139,149] Planar regenerative scaffold electrodes (RSE) were designed with multiple guidance channels leading to the electrode array.[139] Successful regeneration was observed across the devices with recorded nerve compound action potentials. An advantage to this approach, which represents a major step toward the merger of tissue engineering and electronic neural interfaces, is the demonstration of robust regeneration onto a sheet integrated into a semi-permeable polysulfone tube that is sutured to the ends of a cut nerve. A limitation of the work demonstrated to date is that it has only been implemented with a single nanofiber sheet with functional electrodes, although a patent by the same authors envisions a more scalable approach.[150] Also, the approach has only yielded recordings of evoked compound nerve action potentials in terminal preparations using pre-clinical models. Improvements in electrode design, configuration, and scalable number are needed.
4.4. TEENI: Tissue-Engineered Electronic Nerve Interface
Another regenerative approach is the tissue-engineered electronic nerve interface (TEENI) technology developed collaboratively by the authors’ research groups. In this approach, multielectrode polymer-based threads are embedded into tissue-engineered hydrogel nerve bioscaffolds to enable natural nerve regeneration (Figure 7).[151–153] By having the tissue regenerate around the electrodes, nerve tissue can better integrate with the electrodes. The fact that TEENI has more flexible electrodes makes it more mechanically compatible with surrounding tissue, which eliminates the drawbacks associated with rigid electrodes commonly used in other PNI approaches. Although multiple implants of microelectrode arrays can lead to greater channel counts and a wider spatial distribution, the number and density of probes penetrating a single nerve is ultimately limited by surgical implantation procedures to a low number for both. In contrast, since the TEENI regenerative approach does not implant the threads into the nerve individually, it does not have an implantation-based limitation on the number or density of the threads but rather is limited by assembly techniques. This approach is scalable to high channel counts because the threads can potentially be spread throughout a sizable volume when multiple thread sets are stacked. The size and number of thread sets can theoretically be tailored to the size of the target nerve.
One possible implementation of TEENI would consist of multi-electrode polyimide-based threads embedded into a biodegradable hydrogel composite, which is wrapped in a piece of bioresorbable SIS and sutured across the ends of a transected nerve (Figure 7).[140] The hydrogel scaffold holds the polyimide threads in position during implantation and provides an ECM-mimicking environment for tissue regeneration and axon elongation around the electrodes. The TEENI approach also provides ample room for axonal regeneration and maturation over time without space constriction.
5. Key Challenge of Foreign Body Response (FBR)
5.1. Limited Long-Term Reliability with Current Devices
An unresolved challenge for all microelectrode–tissue interfaces is the long-term reliability of chronically implanted neuro-prostheses. As mentioned previously, this has been extensively studied in central nervous system implants; it is instructive to briefly summarize this work. In the central nervous system, the putative mechanism limiting reliability is inadequate biocompatibility because of the reactive tissue response.[154–156] The reactive tissue response is a complex sequence of events that involves many cell types. For penetrating devices, the initial mechanical insertion of devices into the brain penetrates the blood brain barrier, initiating the inflammatory cascade and resulting in edema as well as a release of erythrocytes and macrophages.[157] After the initial penetrating injury, there is a foreign body response in response to the indwelling device. Within one day, activated microglia are observed around the implanted device.[158] Subsequently, activated astrocytes form a “glial sheath” around the device,[159,160] mechanically and electrically isolating the device from the brain. Although the signal cascade is unknown, the reactive tissue response can be typically accompanied by more long-term neuronal death and migration away from the device, i.e., a “neuronal kill-zone”.[159,161] This increased device-neuron distance significantly reduces the efficacy of the neural interfaces.[162]
PNI histological characterization has reported similar challenges with device tissue integration, compared to central neural interfaces. Figure 8 shows the seminal histological characterization of the nerve cuff,[163] FINE,[37] LIFE,[28] TIME,[164,165] USEA,[166] sieve electrodes,[167] and REMI,[168] and have these devices all show various levels of tissue response including encapsulation tissue, morphological abnormalities, decreases in axon density, scar formation, inflammatory reactions, and a presence of hematogenous macrophages near the implant. The cuff electrode in Figure 8 shows a longitudinal section through the proximal end of the cuff where the void left by the cuff and the encapsulation tissue are visible.[163] There is a severe response to the 0.8 mm FINE electrode at 28 days post-implant.[37] Axon density is decreased and most of the axons are thinly myelinated throughout the section. Degenerating axons and myelin debris are present as is abnormal clustering of axons. For the LIFE electrode implanted in the sciatic nerve, anti-choline acetyltransferase and hematoxylin staining of a transverse section following 3 months is shown. The image depicts a mild scar response and a focal, but chronic, inflammatory reaction, which is limited to a small area around the electrode placed in the nerve. Motor axons grouped in fascicles are present, some of them at a short distance from the electrode, as well as the fibrous tissue around the electrodes.[28] TIME electrodes implanted in the tibial and peroneal nerve branches exhibit mild fibrous tissue surrounding the electrode and preservation of nerve structure.[165] The USEA electrode was implanted in sciatic nerve long-term and labeled with MAC387 and β-III tubulin, which shows macrophages around the electrode tips and neural processes outside of the normal fascicular structure.[166] Sieve electrodes implanted in rat sciatic nerve for 6 months and stained with anti-CD11b show reactive hematogenous macrophages in direct contact with the sieve electrode.[167] A REMI electrode implanted in rat sciatic nerve for 60 days labeled for ED1 shows reactive macrophages at the electrode region.[168] A TEENI electrode implanted in rat sciatic nerve for 6 weeks and then stained for macrophages illustrates FBR in a non-regenerative area surrounding the TEENI device.
Figure 8.
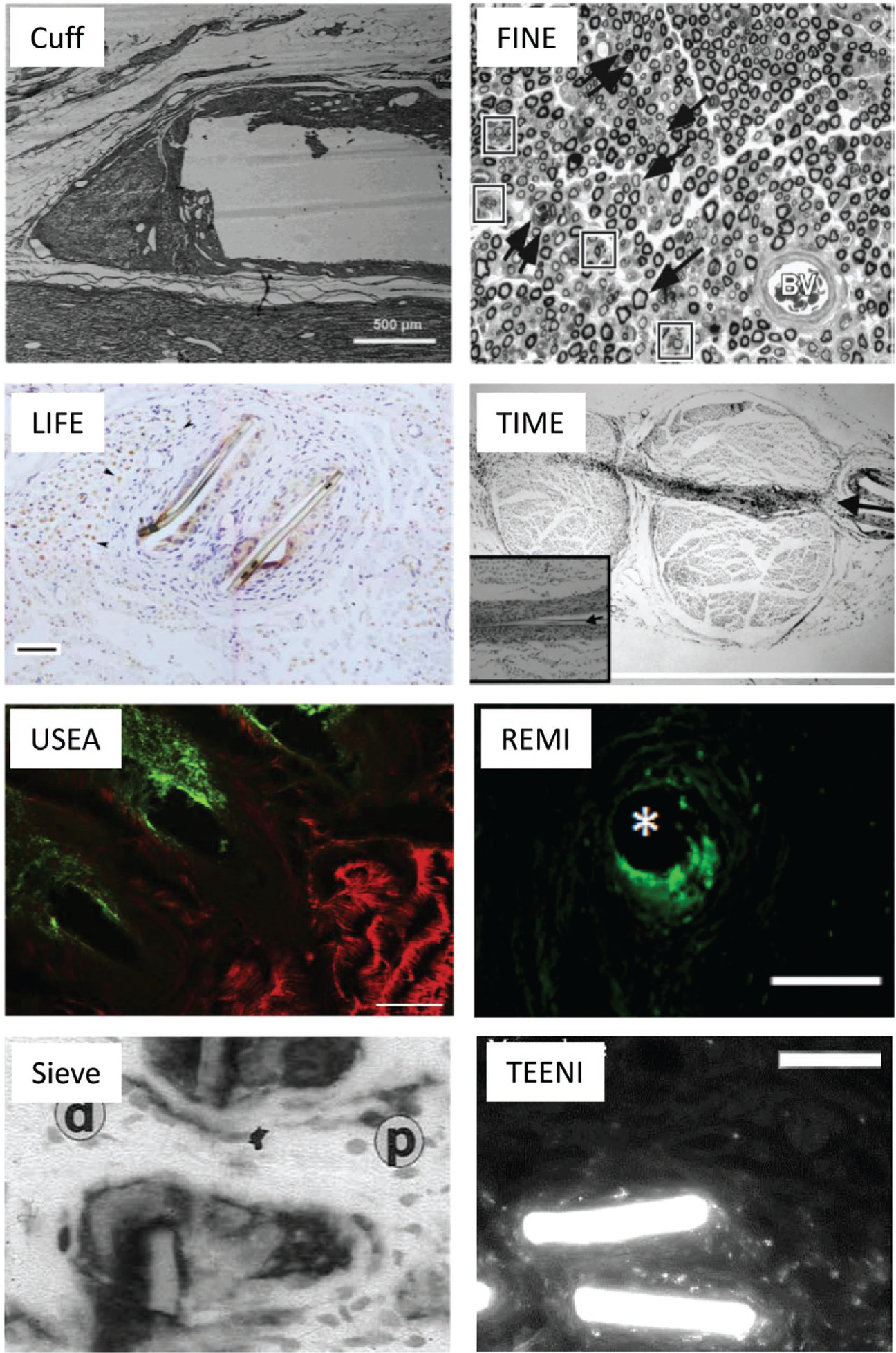
Histological characterization of various PNI devices. Cuff electrode: longitudinal section through the proximal end of the cuff, showing the void left by the cuff, as well as the encapsulation tissue, scale bar = 500 μm. Reproduced with permission.[163] Copyright 2000, Wiley. FINE electrode: severe response after 28 days (methylene blue stain, 40× magnification), arrows point to thin myelination of axons. Degenerating axons and myelin debris are present (double arrows) as is abnormal clustering of axons (boxes); BV = Blood vessel. Reproduced with permission.[37] Copyright 2003, Springer. LIFE electrode: a mild scar response and a focal but chronic inflammatory reaction, which were limited to a small area around the electrode placed in the nerve. Anti-choline acetyltransferase (ChAT) labeling counterstained with hematoxylin (cell nuclei, blue) of a transverse section is shown. Arrowheads point to motor axons (ChAT+, brown), scale bar = 50 μm. Reproduced with permission.[28] Copyright 2007, IEEE. TIME electrode: implanted in sciatic nerve (arrow points to the entrance of the electrode) cross-section shows mild fibrous tissue surrounding the electrode (at higher magnification in the inset; arrowhead points to the polyimide electrode, scale bar = 100 μm). Reproduced with permission.[165] Coypright 2011, IEEE. USEA electrode: after long-term implantation cross-section labeled with MAC387 (green) and β-III tubulin (red) shows macrophages around the electrode tips and neuronal processes outside of the normal fascicular structure, scale bar = 100 μm. Reproduced with permission.[166] Copyright 2014, Elsevier. REMI electrode: the foreign body response immunolabeled with the macrophage label ED1. Macrophages present at explanted electrode region. Asterisk (*) denotes electrode location within the tissue, scale bar; 80 μm. Reproduced with permission.[168] Copyright 2012, the authors. Sieve electrode: transverse section of the sieve-electrode–nerve complex six months after implantation stained with anti-CD11bantibodies. Magnification 390×; p: proximal nerve stump, d: distal neuroma. Reproduced with permission.[167] Copyright 2001, Elsevier. TEENI electrode: cellular component (i.e., macrophages) associated with FBR in non-regenerative area surrounding the TEENI device, scale bar = 50 μm. Reproduced with permission.[153] Copyright 2017 IEEE.
As shown in Figure 8, traditional histological approaches are typically used to evaluate the device-tissue integration. This oftentimes includes removal of the device from the tissue prior to the final imaging preparation. Furthermore, tissue sections are cut very thin, typically ≈10 μm. This allows for adequate label penetration throughout the device, as well as better imaging resolution. However, this approach has several drawbacks, including: a loss of valuable tissue during sectioning, inter-sample variability of labeling and/or imaging, and lengthy tissue preparation and data collection times. Furthermore, typical device–tissue manuscripts do not provide a comprehensive cellular and tissue analysis of the samples (reports often label only a few targets using immunohistochemistry).
More complete understanding of the biochemical and cellular responses to device insertion and indwelling will enable implementation of higher fidelity and reliable interfaces. More recently, investigations were made into alternative cell and tissue markers associated with the device-tissue interface from what is typically used in the field. For example, Graham et al. conducted a thorough evaluation of the TEENI interface, evaluating both neuronal and non-neuronal cells as well as components associated with the extracellular matrix in a regenerative as well as non-regenerative state.[153]
Future histological evaluations of PNIs will likely proceed along two lines. First, fully intact device-tissue interfaces will be investigated by preserving large-scale samples. This will enable optical imaging approaches by using optical clearing technology to render the tissue optically transparent.[169,170] Once the refractive index of the solution matches that of the microscope objective then photon scattering is minimized, even for thick samples (>1 cm). Immunohistochemical labeling of these thick slices is challenging, but is being enabled using electrophoretic approaches.[171] The second approach, in vivo imaging, will likely enable the evaluation and development of highly functional PNIs.[172,173] Observing dynamic changes in appropriate biomarkers of efficacy and performance will facilitate design, fabrication, and evaluation of smart, dynamic, and responsive implants and ultimately help with mitigating performance decline caused by FBR.
5.2. Mitigation of Foreign Body Response
The complex molecular and cellular composition of the regenerated neural interface suggests that a multi-tiered mitigation approach utilizing our understanding of the FBR, histology, and electrode functionality is necessary for long-term integration. What is likely needed is a multiscale, multimodal approach to device–tissue integration for regenerative interfaces. Here we describe chemical coatings, surface topography modification, and drug release approaches that may one day be simultaneously employed to enable long-term PNIs.
5.2.1. Chemical Coatings
One novel mitigation strategy for improving the biocompatibility of interfaces is through the modification of interfacial surface chemistries. A variety of techniques have been employed including the direct adhesion of anti-inflammatories such as dexamethasone,[174] anti-biofouling agents like PEGMA,[175] conductive polymers such as polypyrole,[176] and drug eluting carbon nanotubes,[177] and hydrogels,[178,179] with the expectation that these coatings would assist in FBR mitigation.
While adhesion strategies for anti-inflammatories have shown a reduction in the FBR at acute time points, extended release is necessary for chronic implantation. Conductive polymers such as polypyrrole[176] or PEDOT,[180] have been shown to increase the duration of dexamethasone release for months, but these coatings can only be placed on the electrode sites themselves, as the conductive coating will otherwise cause a shorted circuit; while non-conductive polymers do not exhibit this issue, their deposition can greatly increase device impedance.[178] Hydrogels have been shown to be capable of drug elution and even cytokine capture, but in their hydrated form, these coatings are often hundreds of micrometers thick, dramatically increasing the device footprint, ultimately displacing the tissue with which the device is attempting to communicate.
Second-generation coatings employ multiple strategies to assist in this amelioration, functionalized to manipulate conductivity, reactivity, device thickness, young’s modulus, porosity and therapeutic release. Current strategies incorporate drug eluting carbon nanotubes and PEDOT coated electrodes,[181] drug-eluting nanofibers, encapsulated within alginate with PEDOT electrode sites,[178] or extended release layer-by-layer directed drug delivery only nanometers in thickness[182,183] with minimal impact on device performance.[184] Sol–gel coatings also show great promise, exhibiting extended controlled release[185] from thin-film (≈2–4 μm) coatings.[186,187] As they are also capable of seeding with cells,[188] and functionalization[189] without a negative impact on device performance.[185–187]
5.2.2. Surface Topology Modification
A novel approach is a breakthrough discovery by Brennan and colleagues that cellular interaction, and resulting biofouling, can be greatly mitigated by engineering microtopographic cues into surfaces. This technology, which has been commercialized by a spin-out company (Sharklet®) to engineer bacterialgrowth-impeding surfaces for hospitals, has also been shown to induce epithelial cells (keratinocytes) to reform a dermis more rapidly on wounds.[190] The Sharklet® microtopography was developed to demonstrate the ability to use the wettability of the pattern as well as the physical topography to control (i.e., enhance or inhibit) bioadhesion.[191,192]
5.2.3. Drug Release
With the exciting possibilities on the horizon concerning synthetic coatings, great interest is also being given to both traditional anti-inflammatories as well as more novel, biologically relevant therapeutics. Historically, FBR modulating drugs associated with neural implants include cortical steroid dexamethasone and antibiotic/antioxidant minocycline to foster a less inflamed/less reactive tissue interface. As previously mentioned, these drugs have had limited success at acute time points but have failed to mitigate inflammation at more chronic time points (mainly because of their limited duration of release).[174,193] However, by implementing the coating strategies outlined above, a variety of non-traditional macromolecules, such as nerve growth factor and other neurotrophins can be released concurrently and independently. The very nature in which coatings can be applied independently allows for selective coating paradigms capable of programming individual electrodes or segments of the electrode array to recruit motor but not sensory axons and vice versa, while still promoting nerve regeneration and the amelioration of the FBR.
6. Conclusions
In this paper, we have reviewed state-of-the-art peripheral nerve interfaces, remaining challenges in the field, and nerve regeneration approaches that are contributing to future advances in PNI design. Among the many PNIs that have been designed to date, including nerve cuffs, FINE, LIFE, TIME, and USEA, each design has its own unique set of advantages and disadvantages. The ideal PNI design should be able to sense low amplitude electrical signals, which can be facilitated through enhanced physical and electrical connections. In addition, PNIs should exhibit consistent performance over long time periods, requiring optimized PNI and tissue integration to minimize adverse foreign body response.
Recently, there has been increased interest in PNI designs where, instead of electrodes being inserted directly into the nerve or wrapped around the nerve, the nerve is instead transected and allowed to regenerate into the PNI. This approach should promote a better integration of the PNI electrodes with the host nerve tissue. Peripheral nerve tissue engineering is similarly concerned with regenerating peripheral nerve. Therefore, peripheral nerve regeneration strategies that incorporate native extracellular matrix components, natural nerve microarchitectures, and that also replicate the mechanical properties of native nerves are capable of improving PNI-tissue interfacing. We highlight several early attempts of combining neural-interface engineering and tissue engineering, including our tissue-engineered electronic nerve interface (TEENI) design.
However, even taking this approach, challenges remain such as the foreign body response caused by the mechanical, topological, and chemical mismatch between the electrodes and surrounding tissue. This challenge may be mitigated utilizing strategies such as chemical coatings of electrodes, surface topology modification, or combining additional drug releasing systems to the PNIs. Regardless of the challenges, there are many advantages to utilizing a regenerative approach for developing PNIs and ultimately improving performance for amputees.
Acknowledgements
B.S.S., V.H.D., and S.M. contributed equally to this work. We wish to acknowledge Van Truong for artwork for Figures 1, 3, and 4; as well as Christopher Lacko, Andrew Garcia, and Dr. Carlos Rinaldi for allowing us to use their unpublished images in Figure 5D. We also wish to acknowledge our team members who have contributed to conceptual design and research, and as cited in the appropriate publications: Dr. Rebecca Wachs, Dr. Elizabeth Nunamaker, Eric Atkinson, and Chancellor Shafor. Results presented on the TEENI device were funded by the Defense Advanced Research Projects Agency (DARPA) Biological Technologies Office (BTO) Electrical Prescriptions (ElectRx) program under the auspices of Dr. Doug Weber through the DARPA Contracts Management Office, Pacific Cooperative Agreement: No. HR0011-15-2-0030.
Biographies

Kevin J. Otto is currently an Associate Professor in the J. Crayton Pruitt Family Department of Biomedical Engineering at the University of Florida (UF). Prior to UF, he was on the faculty at Purdue University. He received his PhD in Chemical Engineering from Colorado State University and performed Post-Doctoral Training at the University of Michigan. Dr. Otto’s research interests include neural engineering, device–tissue interfaces, and neurostimulation.

Jack W. Judy is the Director of the Nanoscience Institute for Medical and Engineering Technology (NIMET) and holds the Intel Nanotechnology Endowed Chair in Electrical and Computer Engineering at the University of Florida. Dr. Judy was formerly a program manager in the Microsystems Technology Office of the Defense Advanced Research Projects Agency (DARPA). His research interests span a wide range of neuroengineering topics, such as reliable high-performance neural interfaces to the peripheral nervous system; new sensor technologies to advanced deep-brain-stimulation technologies; miniature, implantable, high-speed, multichannel chemical sensors; wireless neural telemetry and packaging; and a variety of biological micro/nano-electromechanical systems (BioMEMS/NEMS).

Christine E. Schmidt is the J. Crayton Pruitt Family Endowed Chair and Department Chair of the J. Crayton Pruitt Family Department of Biomedical Engineering at the University of Florida. Prior to UF, she was on the faculty at the University of Texas at Austin. She received her PhD in Chemical Engineering from the University of Illinois in Urbana Champaign. Dr. Schmidt’s research is focused on developing new biomaterials and biomaterial composites (e.g., natural material scaffolds, processed tissues, electronic polymer composites) for neural regeneration applications. Dr. Schmidt’s research has resulted in a number of patents and clinical translation to bioengineering products.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Contributor Information
Benjamin S. Spearman, Crayton Pruitt Family Department of Biomedical Engineering, The University of Florida, 1275 Center Dr., BMS Building JG-56, 116131, Gainesville, FL 32611-6131
Vidhi H. Desai, Department of Electrical and Computer Engineering, The University of Florida, 216 Larsen Hall, 116200, Gainesville, FL 32611-6200 Nanoscience Institute for Medical and Engineering Technology, The University of Florida, 1041 Center Drive, 116621, Gainesville, FL 32611-6621.
Sahba Mobini, Crayton Pruitt Family Department of Biomedical Engineering, The University of Florida, 1275 Center Dr., BMS Building JG-56, 116131, Gainesville, FL 32611-6131.
Matthew D. McDermott, Crayton Pruitt Family Department of Biomedical Engineering, The University of Florida, 1275 Center Dr., BMS Building JG-56, 116131, Gainesville, FL 32611-6131 Weldon School of Biomedical Engineering, Purdue University, 206 S. Martin Jischke Dr., West Lafayette, IN 47907-2032.
James B. Graham, Crayton Pruitt Family Department of Biomedical Engineering, The University of Florida, 1275 Center Dr., BMS Building JG-56, 116131, Gainesville, FL 32611-6131
Kevin J. Otto, Crayton Pruitt Family Department of Biomedical Engineering, The University of Florida, 1275 Center Dr., BMS Building JG-56, 116131, Gainesville, FL 32611-6131 Nanoscience Institute for Medical and Engineering Technology, The University of Florida, 1041 Center Drive, 116621, Gainesville, FL 32611-6621; Department of Neuroscience, The University of Florida, 1149 Newell Dr., Room L1-100, 100244, Gainesville, FL 32610-0244; Department of Neurology, The University of Florida, 2000 SW Archer Rd., Third Floor, 100383, Gainesville, FL 32610.
Jack W. Judy, Crayton Pruitt Family Department of Biomedical Engineering, The University of Florida, 1275 Center Dr., BMS Building JG-56, 116131, Gainesville, FL 32611-6131 Department of Electrical and Computer Engineering, The University of Florida, 216 Larsen Hall, 116200, Gainesville, FL 32611-6200; Nanoscience Institute for Medical and Engineering Technology, The University of Florida, 1041 Center Drive, 116621, Gainesville, FL 32611-6621.
Christine E. Schmidt, Crayton Pruitt Family Department of Biomedical Engineering, The University of Florida, 1275 Center Dr., BMS Building JG-56, 116131, Gainesville, FL 32611-6131 Nanoscience Institute for Medical and Engineering Technology, The University of Florida, 1041 Center Drive, 116621, Gainesville, FL 32611-6621.
References
- [1].Shealy CN, Mortimer JT, Reswick JB, Anesth. Analg. 1967, 46, 489. [PubMed] [Google Scholar]
- [2].Cameron T, Neurosurg J. Spine 2004, 100, 254. [DOI] [PubMed] [Google Scholar]
- [3].Blomstedt P, Hariz G-M, Hariz MI, Koskinen L-OD, Br. J. Neurosurg. 2007, 21, 504. [DOI] [PubMed] [Google Scholar]
- [4].Benabid AL, Chabardes S, Mitrofanis J, Pollak P, Ziégler M, Gill S, Lancet Neurol. 2009, 8, 67. [DOI] [PubMed] [Google Scholar]
- [5].Vidailhet M, Vercueil L, Houeto J-L, Krystkowiak P, Lagrange C, Yelnik J, Bardinet E, Benabid A-L, Navarro S, Dormont D, Grand S, Blond S, Ardouin C, Pillon B, Dujardin K, Hahn-Barma V, Agid Y, Destée A, Pollak P, The French SPIDY Study Group, Lancet. Neurol. 2007, 6, 223. [DOI] [PubMed] [Google Scholar]
- [6].Judy JW, IEEE Pulse 2012, 3, 57. [DOI] [PubMed] [Google Scholar]
- [7].Microsystems Technology Office (DARPA), Histology for Interface (HIST), https://www.darpa.mil/work-with-us/histology-for-interface-stability-over-time (accessed: August 2017).
- [8].Microsystems Technology Office (DARPA), Reliable Peripheral Interfaces (RPI), https://www.darpa.mil/work-with-us/reliable-peripheral-interfaces (accessed: August 2017).
- [9].Tracey KJ, Sci. Am. 2015, 312, 28. [Google Scholar]
- [10].Famm K, Litt B, Tracey KJ, Boyden ES, Slaoui M, Nature 2013, 496, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ratner M, Nat. Biotechnol. 2014, 32, 608. [DOI] [PubMed] [Google Scholar]
- [12].Biological Technologies Office (DARPA), Hand Proprioception and Touch Interfaces (HAPTIX), https://www.darpa.mil/program/hand-proprioception-and-touch-interfaces (accessed: August 2017).
- [13].McLoughlin MP, presented at MORS Personnel and National Security Workshop, DARPA Revolutionizing Prosthetics 2009, Laurel, MD 2009. [Google Scholar]
- [14].Birmingham K, Gradinaru V, Anikeeva P, Grill WM, Pikov V, McLaughlin B, Pasricha P, Weber D, Ludwig K, Famm K, Nat. Rev. Drug Discovery 2014, 13, 399. [DOI] [PubMed] [Google Scholar]
- [15].Sinha S, Prasad GL, Lalwani S, J. Neurosurg. 2015, 125, 1. [DOI] [PubMed] [Google Scholar]
- [16].Brill NA, Tyler DJ, Muscle Nerve 2017, DOI: 10.1002/mus.25534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Delgado-Martínez I, Badia J, Pascual-Font A, Rodríguez-Baeza A, Navarro X, Front. Neurosci. 2016, 10, 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Badia J, Pascual-Font A, Vivó M, Udina E, Navarro X, Muscle Nerve 2010, 42, 192. [DOI] [PubMed] [Google Scholar]
- [19].Salzer JL, Neuron 1997, 18, 843. [DOI] [PubMed] [Google Scholar]
- [20].Desai VH, Evaluation and Use of Regenerative Multi-electrode Interfaces in Peripheral Nerves, University of Texas at Arlington, Arlington, TX: 2014. [Google Scholar]
- [21].Clark GA, Ledbetter NM, Warren DJ, Harrison RR, Conf. Proc. IEEE Eng. Med. Biol. Soc. 2011, 2011, 4641. [DOI] [PubMed] [Google Scholar]
- [22].De Luca CJ, Gilmore LD, Science 1976, 191, 193. [DOI] [PubMed] [Google Scholar]
- [23].Stein R, Nichols T, Jhamandas J, Brain Res. 1977, 128, 21. [DOI] [PubMed] [Google Scholar]
- [24].Rodríguez FJ, Ceballos D, Schüttler M, Valero A, Valderrama E, Stieglitz T, Navarro X, Neurosci J. Methods 2000, 98, 105. [DOI] [PubMed] [Google Scholar]
- [25].Ordonez JS, Pikov V, Wiggins H, Patten C, Stieglitz T, Rickert J, Schuettler M, in 2014 36th Annual Int. Conf. of the IEEE Engineering in Medicine and Biology Society, IEEE, Chicago, IL: 2014, pp. 6846–6849. [DOI] [PubMed] [Google Scholar]
- [26].Ardiem Medical, Neural Cuff | Ardiem Medical, www.ardiemmedical.com (accessed: May 2017).
- [27].Schiefer MA, Freeberg M, Pinault GJC, Anderson J, Hoyen H, Tyler DJ, Triolo RJ, J. Neural Eng. 2013, 10, 56006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lago N, Yoshida K, Koch KP, Navarro X, IEEE Trans. Biomed. Eng. 2007, 54, 281. [DOI] [PubMed] [Google Scholar]
- [29].Hassler C, Boretius T, Stieglitz T, J. Polym. Sci. Part B Polym. Phys. 2011, 49, 18. [Google Scholar]
- [30].Wark HAC, Sharma R, Mathews KS, Fernandez E, Yoo J, Christensen B, Tresco P, Rieth L, Solzbacher F, Normann RA, Tathireddy P, J. Neural Eng 2013, 10, 45003. [DOI] [PubMed] [Google Scholar]
- [31].Jeong J, Jung W, Kim O, Chu JU, Youn I, Kim K, Oh SR, Park JW, Kim J, in Proc. IEEE RAS EMBS Int. Conf. Biomed. Robot. Biomechatronics, IEEE, Singapore 2016, 1148. [Google Scholar]
- [32].Srinivasan A, Tahilramani M, Bentley JT, Gore RK, Millard DC, Mukhatyar VJ, Joseph A, Haque AS, Stanley GB, English AW, Bellamkonda RV, Biomaterials 2015, 41, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kim Y, Romero-Ortega MI, MRS Bull. 2012, 37, 573. [Google Scholar]
- [34].De Luca CJ, Gilmore LD, Bloom LJ, Thomson SJ, Cudworth AL, Glimcher MJ, IEEE Trans. Biomed. Eng. 1982, 29, 393. [DOI] [PubMed] [Google Scholar]
- [35].Loeb GE, Peck RA, J. Neurosci. Methods 1996, 64, 95. [DOI] [PubMed] [Google Scholar]
- [36].Tyler DJ, Durand DM, IEEE Trans. Neural Syst. Rehabil. Eng. 2002, 10, 294. [DOI] [PubMed] [Google Scholar]
- [37].Tyler DJ, Durand DM, Ann. Biomed. Eng. 2003, 31, 633. [DOI] [PubMed] [Google Scholar]
- [38].Tan DW, Schiefer MA, Keith MW, Anderson JR, Tyler J, Tyler DJ, Sci. Transl. Med. 2014, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wodlinger B, Durand DM, J. Neural Eng 2011, 8, 56005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Micera S, Navarro X, Carpaneto J, Citi L, Tonet O, Rossini PM, Carrozza MC, Hoffmann KP, Vivó M, Yoshida K, Dario P, IEEE Trans. Neural Syst. Rehabil. Eng. 2008, 16, 453. [DOI] [PubMed] [Google Scholar]
- [41].Micera S, Rossini PM, Rigosa J, Citi L, Carpaneto J, Raspopovic S, Tombini M, Cipriani C, Assenza G, Carrozza MC, Hoffmann K-P, Yoshida K, Navarro X, Dario P, J. Neuroeng. Rehabil. 2011, 8, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rossini PM, Micera S, Benvenuto A, Carpaneto J, Cavallo G, Citi L, Cipriani C, Denaro L, Denaro V, Di Pino G, Ferreri F, Guglielmelli E, Hoffmann K-P, Raspopovic S, Rigosa J, Rossini L, Tombini M, Dario P, Clin. Neurophysiol. 2010, 121, 777. [DOI] [PubMed] [Google Scholar]
- [43].Citi L, Carpaneto J, Yoshida K, Hoffmann K-P, Koch KP, Dario P, Micera S, J. Neurosci. Methods 2008, 172, 294. [DOI] [PubMed] [Google Scholar]
- [44].Yoshida K, Stein RB, IEEE Trans. Biomed. Eng. 1999, 46, 226. [DOI] [PubMed] [Google Scholar]
- [45].Ma Z, Hu S, Tan JS, Myer C, Njus NM, Xia Z, J. Biomed. Mater. Res. – Part A 2013, 101A, 2718. [DOI] [PubMed] [Google Scholar]
- [46].Lacour SP, Courtine G, Guck J, Deisseroth K, Delp SL, Nat. Rev. Mater. 2016, 1, 16063. [Google Scholar]
- [47].Lefurge T, Goodall E, Horch K, Ann. Biomed. Eng. 1991, 19, 197. [DOI] [PubMed] [Google Scholar]
- [48].Lawrence SM, Dhillon GS, Jensen W, Yoshida K, Horch KW, IEEE Trans. Neural Syst. Rehabil. Eng. 2004, 12, 345. [DOI] [PubMed] [Google Scholar]
- [49].Yoshida K, Pellinen D, Piven D, Rousche PJ, Kipke DR, in Proc. 5th Annu. Conf. IFESS, Sheffield, UK 2000, pp. 1497–1500. [Google Scholar]
- [50].Boretius T, Badia J, Pascual-Font A, Schuettler M, Navarro X, Yoshida K, Stieglitz T, Biosens. Bioelectron. 2010, 26, 62. [DOI] [PubMed] [Google Scholar]
- [51].Boretius T, Yoshida K, Badia J, Harreby K, Kundu A, Navarro X, Jensen W, Stieglitz T, 2012 4th IEEE RAS EMBS Int. Conf. Biomed. Robot. Biomechatronics 2012, 282. [Google Scholar]
- [52].Raspopovic S, Capogrosso M, Petrini FM, Bonizzato M, Rigosa J, Di Pino G, Carpaneto J, Controzzi M, Boretius T, Fernandez E, Granata G, Oddo CM, Citi L, Ciancio AL, Cipriani C, Carrozza MC, Jensen W, Guglielmelli E, Stieglitz T, Rossini PM, Micera S, Sci. Transl. Med. 2014, 6, 222. [DOI] [PubMed] [Google Scholar]
- [53].Campbell PK, Jones KE, Huber RJ, Horch KW, Normann RA, IEEE Trans. Biomed. Eng. 1991, 38, 758. [DOI] [PubMed] [Google Scholar]
- [54].Jones KE, Campbell PK, Normann RA, Ann. Biomed. Eng. 1992, 20, 423. [DOI] [PubMed] [Google Scholar]
- [55].Nordhausen CT, Maynard EM, Normann RA, Brain Res. 1996, 726, 129. [PubMed] [Google Scholar]
- [56].Branner A, Normann RA, Brain Res. Bull. 2000, 51, 293. [DOI] [PubMed] [Google Scholar]
- [57].Davis TS, Wark HAC, Hutchinson DT, Warren DJ, O’Neill K, Scheinblum T, Clark GA, Normann RA, Greger B, J. Neural Eng 2016, 13, 36001. [DOI] [PubMed] [Google Scholar]
- [58].Clark GA, Wendelken S, Page DM, Davis T, Wark HAC, Normann RA, Warren DJ, Hutchinson DT, in 2014 36th Annual Int. Conf. of the IEEE Engineering in Medicine and Biology Society, IEEE, Chicago, IL 2014, pp. 1977–1980. [DOI] [PubMed] [Google Scholar]
- [59].Xie X, Rieth L, Williams L, Negi S, Bhandari R, Caldwell R, Sharma R, Tathireddy P, Solzbacher F, J. Neural Eng 2014, 11, 26016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Barrese JC, Rao N, Paroo K, Triebwasser C, Vargas-Irwin C, Franquemont L, Donoghue JP, J. Neural Eng 2013, 10, 66014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Robinson LR, Muscle Nerve 2000, 23, 863. [DOI] [PubMed] [Google Scholar]
- [62].Sunderland S, Nerves and Nerve Injury, 1st ed., E. & S. Livingstone Edinburgh, Livingstone, Edinburgh: 1978. [Google Scholar]
- [63].Kovacs GT, Storment CW, Rosen JM, IEEE Trans. Biomed. Eng. 1992, 39, 893. [DOI] [PubMed] [Google Scholar]
- [64].Mannard A, Stein RB, Charles D, Science 1974, 183, 547. [DOI] [PubMed] [Google Scholar]
- [65].Edell DJ, IEEE Trans. Biomed. Eng. 1986, 33, 203. [DOI] [PubMed] [Google Scholar]
- [66].Rosen JM, Grosser M, Hentz VR, Restor. Neurol. Neurosci. 1990, 2, 89. [DOI] [PubMed] [Google Scholar]
- [67].Della Santina CC, Kovacs GT, Lewis ER, J. Neurosci. Methods 1997, 72, 71. [DOI] [PubMed] [Google Scholar]
- [68].Bradley RM, Smoke RH, Akin T, Najafi K, Brain Res. 1992, 594, 84. [DOI] [PubMed] [Google Scholar]
- [69].Akin T, Najafi K, Smoke RH, Bradley RM, IEEE Trans. Biomed. Eng. 1994, 41, 305. [DOI] [PubMed] [Google Scholar]
- [70].Najafi K, IEEE Eng. Med. Biol. Mag. 1994, 13, 375. [Google Scholar]
- [71].Bradley RM, Cao X, Akin T, Najafi K, J. Neurosci. Methods 1997, 73, 177. [DOI] [PubMed] [Google Scholar]
- [72].Mensinger AF, Anderson DJ, Buchko CJ, Johnson MA, Martin DC, a Tresco P, Silver RB, Highstein SM, J. Neurophysiol 2000, 83, 611. [DOI] [PubMed] [Google Scholar]
- [73].Blau A, Ziegler C, Heyer M, Endres F, Schwitzgebel G, Matthies T, Stieglitz T, Meyer JU, Göpel W, Biosens. Bioelectron. 1997, 12, 883. [DOI] [PubMed] [Google Scholar]
- [74].Stieglitz T, Beutel H, Meyer J-U, Sensors Actuators A Phys. 1997, 60, 240. [Google Scholar]
- [75].Shimatani Y, Nikles SA, Najafi K, Bradley RM, Physiol. Behav. 2003, 80, 309. [DOI] [PubMed] [Google Scholar]
- [76].Ramachandran A, Schuettler M, Lago N, Doerge T, Koch KP, Navarro X, Hoffmann K-P, Stieglitz T, J. Neural Eng 2006, 3, 114. [DOI] [PubMed] [Google Scholar]
- [77].Lago N, Udina E, Ramachandran A, Navarro X, IEEE Trans. Biomed. Eng. 2007, 54, 1129. [DOI] [PubMed] [Google Scholar]
- [78].Lago N, Ceballos D, Rodríguez FJ, Stieglitz T, Navarro X, Biomaterials 2005, 26, 2021. [DOI] [PubMed] [Google Scholar]
- [79].Castro J, Negredo P, Avendaño C, Brain Res. 2008, 1190, 65. [DOI] [PubMed] [Google Scholar]
- [80].Negredo P, Castro J, Lago N, Navarro X, Avendaño C, Neuroscience 2004, 128, 605. [DOI] [PubMed] [Google Scholar]
- [81].Marks A, Anat. Rec. 1969, 163, 226. [Google Scholar]
- [82].Loeb GE, Marks WB, Beatty PG, Med. Biol. Eng. Comput. 1977, 15, 195. [DOI] [PubMed] [Google Scholar]
- [83].Matsuo T, Yamaguchi A, Esashi M, J. Japan Soc ME BE 1978. [Google Scholar]
- [84].FitzGerald JJ, Lacour SP, McMahon SB, Fawcett JW, IEEE Trans. Biomed. Eng. 2008, 55, 1136. [DOI] [PubMed] [Google Scholar]
- [85].FitzGerald JJ, Lago N, Benmerah S, Serra J, Watling CP, Cameron RE, Tarte E, Lacour SP, McMahon SB, Fawcett JW, J. Neural Eng 2012, 9, 16010. [DOI] [PubMed] [Google Scholar]
- [86].Minev IR, Chew DJ, Delivopoulos E, Fawcett JW, Lacour SP, J. Neural Eng 2012, 9, 26005. [DOI] [PubMed] [Google Scholar]
- [87].Srinivasan A, Guo L, Bellamkonda RV, 2011 5th Int. IEEE/EMBS Conf. Neural Eng. (NER), IEEE, Cancun, Mexico 2011, 253. [Google Scholar]
- [88].Delivopoulos E, Chew DJ, Minev IR, Fawcett W, Lacour SP, Lab Chip 2012, 12, 2540. [DOI] [PubMed] [Google Scholar]
- [89].Gore RK, Choi Y, Bellamkonda R, English A, J. Neural Eng 2015, 12, 16017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Musick KM, Rigosa J, Narasimhan S, Wurth S, Capogrosso M, Chew DJ, Fawcett JW, Micera S, Lacour SP, Sci. Rep. 2015, 5, 14363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Srinivasan A, Tipton J, Tahilramani M, Kharbouch A, Gaupp E, Song C, Venkataraman P, Falcone J, Lacour SP, Stanley GB, English AW, Bellamkonda RV, Eur. J. Neurosci. 2016, 43, 474. [DOI] [PubMed] [Google Scholar]
- [92].Freeman MR, Curr. Opin. Neurobiol. 2014, 27, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Seddon HJ, Br. Med. J. 1942, 2, 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Schmidt CE, Leach JB, Annu. Rev. Biomed. Eng. 2003, 5, 293. [DOI] [PubMed] [Google Scholar]
- [95].Pabari A, Lloyd-Hughes H, Seifalian AM, Mosahebi A, Plast. Reconstr. Surg. 2014, 133, 1420. [DOI] [PubMed] [Google Scholar]
- [96].Ray WZ, Mackinnon SE, Exp. Neurol. 2010, 223, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Arslantunali D, Dursun T, Yucel D, Hasirci N, Hasirci V, Med. Devices Evid. Res. 2014, 7, 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Pinho AC, Fonseca AC, Serra AC, Santos JD, Coelho JFJ, Adv. Healthcare Mater. 2016, 5, 2732. [DOI] [PubMed] [Google Scholar]
- [99].Okamoto H, Hata KI, Kagami H, Okada K, Ito Y, Narita Y, Hirata H, Sekiya I, Otsuka T, Ueda M, J. Biomed. Mater. Res. – Part A 2010, 92, 859. [DOI] [PubMed] [Google Scholar]
- [100].Tonda-Turo C, Audisio C, Gnavi S, Chiono V, Gentile P, Raimondo S, Geuna S, Perroteau I, Ciardelli G, Adv. Eng. Mater. 2011, 13, 151. [Google Scholar]
- [101].Yao L, de Ruiter GCW, Wang H, Knight AM, Spinner RJ, Yaszemski MJ, Windebank AJ, Pandit A, Biomaterials 2010, 31, 5789. [DOI] [PubMed] [Google Scholar]
- [102].Belanger K, Dinis TM, Taourirt S, Vidal G, Kaplan DL, Egles C, Macromol. Biosci. 2016, 16, 472. [DOI] [PubMed] [Google Scholar]
- [103].Galla T, Vedecnik S, Halbgewach J, Steinmann S, Friedrich C, Stark G, Int. J. Artif. Organs 2004, 27, 127. [DOI] [PubMed] [Google Scholar]
- [104].Oliveira JT, Bittencourt-Navarrete RE, de Almeida FM, Tonda-Turo C, Martinez AMB, Franca JG, Front. Neuroanat. 2014, 8, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Georgiou M, Golding JP, Loughlin AJ, Kingham PJ, Phillips JB, Biomaterials 2015, 37, 242. [DOI] [PubMed] [Google Scholar]
- [106].Gonzalez-Perez F, Udina E, Navarro X, in Int. Rev. Neurobiology, Vol. 108, Academic Press, Cambridge, MA: 2013, pp. 257–275. [DOI] [PubMed] [Google Scholar]
- [107].Szynkaruk M, Kemp SWP, Wood MD, Gordon T, Borschel GH, Tissue Eng. Part B. Rev. 2013, 19, 83. [DOI] [PubMed] [Google Scholar]
- [108].Kehoe S, Zhang XF, Boyd D, Injury 2012, 43, 553. [DOI] [PubMed] [Google Scholar]
- [109].Tian L, Prabhakaran MP, Ramakrishna S, Regen. Biomater. 2015, 2, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Chiono V, Tonda-Turo C, Prog. Neurobiol. 2015, 131, 87. [DOI] [PubMed] [Google Scholar]
- [111].Hu X, Huang J, Ye Z, Xia L, Li M, Lv B, Shen X, Luo Z, Tissue Eng. Part A 2009, 15, 3297. [DOI] [PubMed] [Google Scholar]
- [112].Cao J, Sun C, Zhao H, Xiao Z, Chen B, Gao J, Zheng T, Wu W, Wu S, Wang J, Dai J, Biomaterials 2011, 32, 3939. [DOI] [PubMed] [Google Scholar]
- [113].Thomas RC, Chung PE, Modi SP, Hardy JG, Schmidt CE, Eur. Polym. J. 2017, 87, 487. [Google Scholar]
- [114].Ribeiro-Resende VT, Koenig B, Nichterwitz S, Oberhoffner S, Schlosshauer B, Biomaterials 2009, 30, 5251. [DOI] [PubMed] [Google Scholar]
- [115].Wang K-K, Nemeth IR, Seckel BR, Chakalis-Haley DP, Swann DA, Kuo J-W, Bryan DJ, Cetrulo CL, Microsurgery 1998, 18, 270. [DOI] [PubMed] [Google Scholar]
- [116].Satou T, Nishida S, Hiruma S, Tanji K, Takahashi M, Fujita S, Mizuhara Y, Akai F, Hashimoto S, Pathol. Int. 1986, 36, 199. [DOI] [PubMed] [Google Scholar]
- [117].Madison RD, Da Silva CF, Dikkes P, Brain Res. 1988, 447, 325. [DOI] [PubMed] [Google Scholar]
- [118].Koopmans G, Hasse B, Sinis N, in Int. Rev. Neurobiology, Vol. 87, 2009, pp. 363–379. [DOI] [PubMed] [Google Scholar]
- [119].Chamberlain LJ, Yannas IV, Arrizabalaga A, Hsu HP, Norregaard TV, Spector M, Biomaterials 1998, 19, 1393. [DOI] [PubMed] [Google Scholar]
- [120].Ceballos D, Navarro X, Dubey N, Wendelschafer-Crabb G, Kennedy WR, Tranquillo RT, Exp. Neurol. 1999, 158, 290. [DOI] [PubMed] [Google Scholar]
- [121].Armstrong SJ, Wiberg M, Terenghi G, Kingham PJ, Tissue Eng. 2007, 13, 2863. [DOI] [PubMed] [Google Scholar]
- [122].Bailey SB, Eichler ME, Villadiego A, Rich KM, J. Neurocytol. 1993, 22, 176. [DOI] [PubMed] [Google Scholar]
- [123].Mosahebi A, Wiberg M, Terenghi G, Tissue Eng. 2003, 9, 209. [DOI] [PubMed] [Google Scholar]
- [124].Chen YS, Hsieh CL, Tsai CC, Chen TH, Cheng WC, Hu CL, Yao CH, Biomaterials 2000, 21, 1541. [DOI] [PubMed] [Google Scholar]
- [125].Discher DE, Janmey P, Wang Y-L, Science 2005, 310, 1139. [DOI] [PubMed] [Google Scholar]
- [126].Engler AJ, Sen S, Sweeney HL, Discher DE, Cell 2006, 126, 677. [DOI] [PubMed] [Google Scholar]
- [127].Borschel GH, Kia KF, Kuzon WM, Dennis RG, J. Surg. Res. 2003, 114, 133. [DOI] [PubMed] [Google Scholar]
- [128].Willits RK, Skornia SL, J. Biomater. Sci. Polym. Ed. 2004, 15, 1521. [DOI] [PubMed] [Google Scholar]
- [129].Gu Y, Ji Y, Zhao Y, Liu Y, Ding F, Gu X, Yang Y, Biomaterials 2012, 33, 6672. [DOI] [PubMed] [Google Scholar]
- [130].Rosso G, Liashkovich I, Young P, Röhr D, Shahin V, Nanomed. Nanotechnol., Biol. Med 2016, 1. [Google Scholar]
- [131].Ingber DE, Curr. Opin. Cell Biol. 1991, 3, 841. [DOI] [PubMed] [Google Scholar]
- [132].Letourneau PC, Shattuck TA, Development 1989, 105, 505. [DOI] [PubMed] [Google Scholar]
- [133].Winslow BD, Tresco PA, Biomaterials 2010, 31, 1558. [DOI] [PubMed] [Google Scholar]
- [134].Sommakia S, Lee HC, Gaire J, Otto KJ, Curr. Opin. Solid State Mater. Sci. 2014, 18, 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Ghezzi D, Front. Neurosci. 2015, 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Seifert JL, Desai V, Watson RC, Musa T, Kim Y-T, Keefer EW, Romero MI, IEEE Trans. Neural Syst. Rehabil. Eng. 2012, 20, 220. [DOI] [PubMed] [Google Scholar]
- [137].Musallam S, Bak MJ, Troyk PR, Andersen RA, Neurosci J. Methods 2007, 160, 122. [DOI] [PubMed] [Google Scholar]
- [138].Desai VH, Anand S, Tran M, Kanneganti A, Vasudevan S, Seifert JL, Cheng J, Keefer EW, Romero-Ortega MI, in 2014 36th Annual Int. Conf. IEEE Engineering in Medicine and Biology Society, IEEE, Chicago, IL 2014, pp. 1973–1976. [DOI] [PubMed] [Google Scholar]
- [139].Clements IP, Mukhatyar VJ, Srinivasan A, Bentley JT, Andreasen DS, Bellamkonda RV, IEEE Trans. Neural Syst. Rehabil. Eng. 2013, 21, 554. [DOI] [PubMed] [Google Scholar]
- [140].Desai V, Shafor C, Spearman B, Wachs R, Graham J, Atkinson E, Nunamaker E, Schmidt C, Otto KJ, Judy J, 2016 38th Annu. Int. Conf. IEEE Eng. Med. Biol. Soc., IEEE, Orlando, FL 2016. [Google Scholar]
- [141].Kuiken TA, Dumanian GA, Lipschutz RD, Miller LA, Stubblefield KA, Prosthet. Orthot. Int. 2004, 28, 245. [DOI] [PubMed] [Google Scholar]
- [142].Miller LA, Lipschutz RD, Stubblefield KA, Lock BA, Huang H, Williams TW, Weir RF, Kuiken TA, Arch. Phys. Med. Rehabil. 2008, 89, 2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Kuiken TA, Miller LA, Lipschutz RD, Lock BA, Stubblefield K, Marasco PD, Zhou P, Dumanian GA, Lancet 2007, 369, 371. [DOI] [PubMed] [Google Scholar]
- [144].Kung TA, Langhals NB, Martin DC, Johnson PJ, Cederna PS, Urbanchek MG, Plast. Reconstr. Surg. 2014, 133, 1380. [DOI] [PubMed] [Google Scholar]
- [145].Urbanchek MG, Wei B, Baghmanli Z, Sugg K, Cederna PS, Plast. Reconstr. Surg. 2011, 128, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Langhals NB, Woo SL, Moon JD, Larson JV, Leach MK, Cederna PS, Urbanchek MG, 2014 36th Annual Int. Conf. IEEE Engineering in Medicine and Biology Society, IEEE, Chicago, IL 2014, 1989. [DOI] [PubMed] [Google Scholar]
- [147].Irwin ZT, Schroeder KE, Vu PP, Tat DM, Bullard AJ, Woo SL, Sando IC, Urbanchek MG, Cederna PS, Chestek CA, J. Neural Eng. 2016, 13, 46007. [DOI] [PubMed] [Google Scholar]
- [148].Ursu D, Nedic A, Urbanchek M, Cederna P, Gillespie RB, J. Neuroeng. Rehabil. 2017, 14, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Clements IP, Kim YT, Andreasen D, Bellamkonda RV, Proc. 3rd Int. IEEE EMBS Conf. Neural Eng., IEEE, Kohala Coast, HI 2007, 390. [Google Scholar]
- [150].Bellamkonda R, Andreasen D, Clements I, Kim Y-T, US 20100211172 A1 2010.
- [151].Desai VH, , Spearman BS, Shafor CS, Natt S, Teem B, Graham JB, Atkinson EW, Wachs RA, Nunamaker EA, Otto KJ, Schmidt CE, Judy JW, 8th Int. IEEE/EMBS Conf. Neural Eng., IEEE, Shanghai, China 2017. [Google Scholar]
- [152].Nunamaker EA, Spearman BS, Graham JB, Atkinson EW, Desai VH, Shafor CS, Natt S, Wachs RA, Schmidt CE, Judy JW, Otto KJ, 8th Int. IEEE/EMBS Conf. Neural Eng., IEEE, Shanghai, China 2017. [Google Scholar]
- [153].Graham JB, Atkinson EW, Nunamaker EA, Spearman BS, Desai VH, Shafor CS, Natt S, Wachs RA, Schmidt CE, Judy JW, Otto KJ, 8th Int. IEEE/EMBS Conf. Neural Eng., IEEE, Shanghai, China 2017. [Google Scholar]
- [154].Maynard EM, Nordhausen CT, Normann RA, Electroencephalogr. Clin. Neurophysiol. 1997, 102, 228. [DOI] [PubMed] [Google Scholar]
- [155].Shain W, Spataro L, Dilgen J, Haverstick K, Retterer S, Isaacson M, Saltzman M, Turner JN, IEEE Trans. Neural Syst. Rehabil. Eng. 2003, 11, 186. [DOI] [PubMed] [Google Scholar]
- [156].Stensaas SS, Stensaas LJ, Acta Neuropathol. 1978, 41, 145. [DOI] [PubMed] [Google Scholar]
- [157].Schmidt S, Horch K, Normann R, J. Biomed. Mater. Res. 1993, 27, 1393. [DOI] [PubMed] [Google Scholar]
- [158].Szarowski DH, Andersen MD, Retterer S, Spence AJ, Isaacson M, Craighead HG, Turner JN, Shain W, Brain Res. 2003, 983, 23. [DOI] [PubMed] [Google Scholar]
- [159].Edell DJ, Toi VV, McNeil VM, Clark LD, IEEE Trans. Biomed. Eng. 1992, 39, 635. [DOI] [PubMed] [Google Scholar]
- [160].Turner JN, Shain W, Szarowski DH, Andersen M, Martins S, Isaacson M, Craighead HG, Exp. Neurol. 1999, 156, 33. [DOI] [PubMed] [Google Scholar]
- [161].Biran R, Martin DC, Tresco PA, Exp. Neurol. 2005, 195, 115. [DOI] [PubMed] [Google Scholar]
- [162].Grill WM, Nicolelis MAL, Simon SA, in Indwelling Neural Implants: Strategies for Contending with the In vivo Enviornment (Ed: Reichert WM), CRC Press, Boca Raton, FL: 2008, pp. 41–62. [PubMed] [Google Scholar]
- [163].Grill WM, Mortimer JT, J. Biomed. Mater. Res. 2000, 50, 215. [DOI] [PubMed] [Google Scholar]
- [164].Micera S, Sergi PN, Carpaneto J, Citi L, Bossi S, Koch KP, Hoffmann KP, Menciassi A, Yoshida K, Dario P, in Annual International Conference of the IEEE Engineering in Medicine and Biology – Proceedings, IEEE, New York City, NY 2006, pp. 2940–2943. [DOI] [PubMed] [Google Scholar]
- [165].Badia J, Boretius T, Pascual-Font A, Udina E, Stieglitz T, Navarro X, IEEE Trans. Biomed. Eng. 2011, 58, 2324. [DOI] [PubMed] [Google Scholar]
- [166].Christensen MB, Pearce SM, Ledbetter NM, Warren DJ, Clark GA, Tresco PA, Acta Biomater. 2014, 10, 4650. [DOI] [PubMed] [Google Scholar]
- [167].Klinge PM, Vafa MA, Brinker T, Brandis A, Walter GF, Stieglitz T, Samii M, Wewetzer K, Biomaterials 2001, 22, 2333. [DOI] [PubMed] [Google Scholar]
- [168].Useche CAS, Characterization of Immune Response and Peripheral Nerve Regeneration of Implanted Regenerative Multi-Electrode Interface Post-Subchronic Amputation, University of Texas at Arlington, Arlington, TX: 2012. [Google Scholar]
- [169].Chung K, Deisseroth K, Nat. Methods 2013, 10, 508. [DOI] [PubMed] [Google Scholar]
- [170].Ke M-T, Fujimoto S, Imai T, Nat. Neurosci. 2013, 16, 1154. [DOI] [PubMed] [Google Scholar]
- [171].Kim S-Y, Cho JH, Murray E, Bakh N, Choi H, Ohn K, Ruelas L, Hubbert A, McCue M, Vassallo SL, Keller PJ, Chung K, Proc. Natl. Acad. Sci. USA 2015, 112, E6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Svoboda K, Denk W, Kleinfeld D, Tank DW, Nature 1997, 385, 161. [DOI] [PubMed] [Google Scholar]
- [173].Ghosh KK, Burns LD, Cocker ED, Nimmerjahn A, Ziv Y, El Gamal A, Schnitzer MJ, Nat. Methods 2011, 8, 871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Zhong Y, Bellamkonda RV, Brain Res. 2007, 1148, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Kozai TDY, Langhals NB, Patel PR, Deng X, Zhang H, Smith KL, Lahann J, Kotov NA, Kipke DR, Nat. Mater. 2012, 11, 1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Wadhwa R, Lagenaur CF, Cui XT, J. Controlled Release 2006, 110, 531. [DOI] [PubMed] [Google Scholar]
- [177].Luo X, Matranga C, Tan S, Alba N, Cui XT, Biomaterials 2011, 32, 6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Abidian MR, Martin DC, Adv. Funct. Mater. 2009, 19, 573. [Google Scholar]
- [179].Skousen JL, Bridge MJ, Tresco PA, Biomaterials 2015, 36, 33. [DOI] [PubMed] [Google Scholar]
- [180].Boehler C, Kleber C, Martini N, Xie Y, Dryg I, Stieglitz T, Hofmann UG, Asplund M, Biomaterials 2017, 129, 176. [DOI] [PubMed] [Google Scholar]
- [181].Kozai TDY, Catt K, Du Z, Na K, Srivannavit O, Haque RM, Seymour J, Wise KD, Yoon E, Cui XT, IEEE Trans. Biomed. Eng. 2016, 63, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Zhang Z, Li Q, Han L, Zhong Y, Biomed. Mater. 2015, 10, 55006. [DOI] [PubMed] [Google Scholar]
- [183].Zhang Z, Nong J, Zhong Y, J. Neural Eng. 2015, 12, 46015. [DOI] [PubMed] [Google Scholar]
- [184].McDermott MD, Olczak KP, Otto KJ, in Conf. Proc. IEEE Eng. Med. Biol. Soc., IEEE, Jeju Island, Korea 2017. [Google Scholar]
- [185].Pierce AL, Rickus JL, Otto KJ, Sommakia S, Rickus JL, Otto KJ, J. Neurosci. Methods 2009, 180, 106. [DOI] [PubMed] [Google Scholar]
- [186].McDermott MD, Zhang J, Otto KJ, in Proc. 7th Int. IEEE/EMBS Conf. Neural Eng., IEEE, Montpellier, France 2015, 4526, 22. [Google Scholar]
- [187].McDermott MD, Otto KJ, in 2016 38th Annu. Int. Conf. IEEE Eng. Med. Biol. Soc., IEEE, Orlando, FL 2016, 129. [DOI] [PubMed] [Google Scholar]
- [188].Jedlicka SS, McKenzie JL, Leavesley SJ, Little KM, Webster TJ, Robinson JP, Nivens DE, Rickus JL, J. Mater. Chem. 2006, 16, 3221. [Google Scholar]
- [189].Jedlicka SS, Little KM, Nivens DE, Zemlyanov D, Rickus JL, J. Mater. Chem. 2007, 17, 5058. [Google Scholar]
- [190].Magin CM, Neale DB, Drinker MC, Willenberg BJ, Reddy ST, La Perle KM, Schultz GS, Brennan AB, Exp. Biol. Med. 2016, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Decker JT, Kirschner CM, Long CJ, Finlay JA, Callow ME, Callow JA, Brennan AB, Langmuir 2013, 29, 13023. [DOI] [PubMed] [Google Scholar]
- [192].Long CJ, Schumacher JF, Brennan AB, Langmuir 2009, 25, 12982. [DOI] [PubMed] [Google Scholar]
- [193].Spataro L, Dilgen J, Retterer S, Spence AJ, Isaacson M, Turner JN, Shain W, Exp. Neurol. 2005, 194, 289. [DOI] [PubMed] [Google Scholar]


