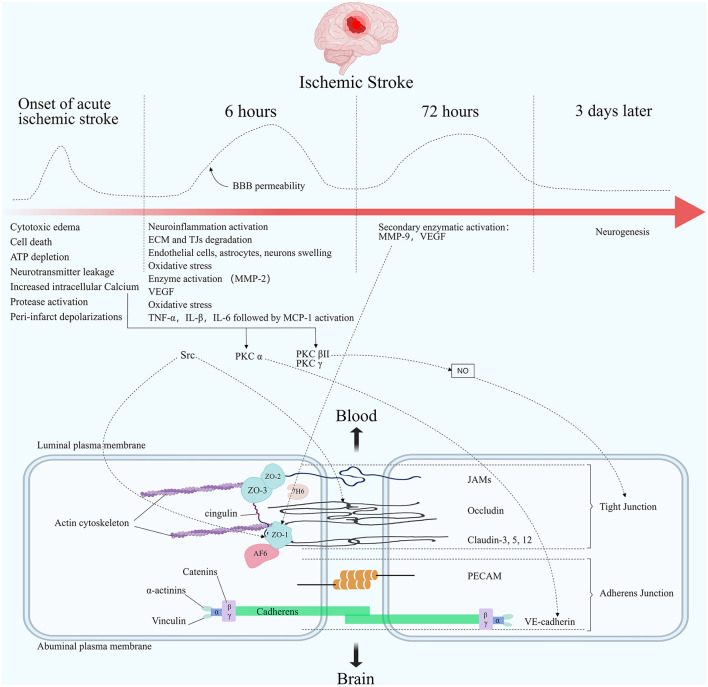
Figure 2.
Molecular composition of BBB tight junctions and major changes in acute ischemic stroke. The TJ consists of three integral membrane proteins, claudins, occludin, and junction adhesion molecules (JAMs), and a number of cytoplasmic accessory proteins including zonula occludens 1, 2, and 3 (ZO-1, 2, 3), cingulin, afadin-6 (AF-6), and 7H6. Membrane proteins are connected to actin by cytoplasmic proteins. The carboxy-terminal serine residues of occludin are linked to the cytoskeleton via ZO-1 and ZO-2. Adherens junctions are formed by cadherins, catenins, vinculin, and actinin. Among the important molecules are vascular endothelial cadherin (VE-cadherin) and platelet endothelial cell adhesion molecules (PECAM). The figure shows the changes in blood-brain barrier permeability at different times after the onset of ischemic stroke, as well as the main pathophysiological processes in each stage. Acutely elevated local cerebral blood flow can lead to initial reperfusion permeability. The opening of the BBB presents a biphasic phenomenon. The first phase usually occurs within 6 h after an acute cerebral infarction. The second phase usually occurs within 72 h after the onset of an acute cerebral infarction. Elevated Ca2+ concentration in the endothelial under ischemic hypoxic conditions regulates TJ proteins' stability through multiple pathways. PKC α can act on VE-cadherin to participate in AJ degradation. PKC βII and PKC γ cause TJ changes by regulating NO activity. Src phosphorylates occludin and ZO-1. Elevated MMP-9 reduces ZO-1 expression and its cytoplasmic translocation, leading to barrier disruption.