Figure 8.
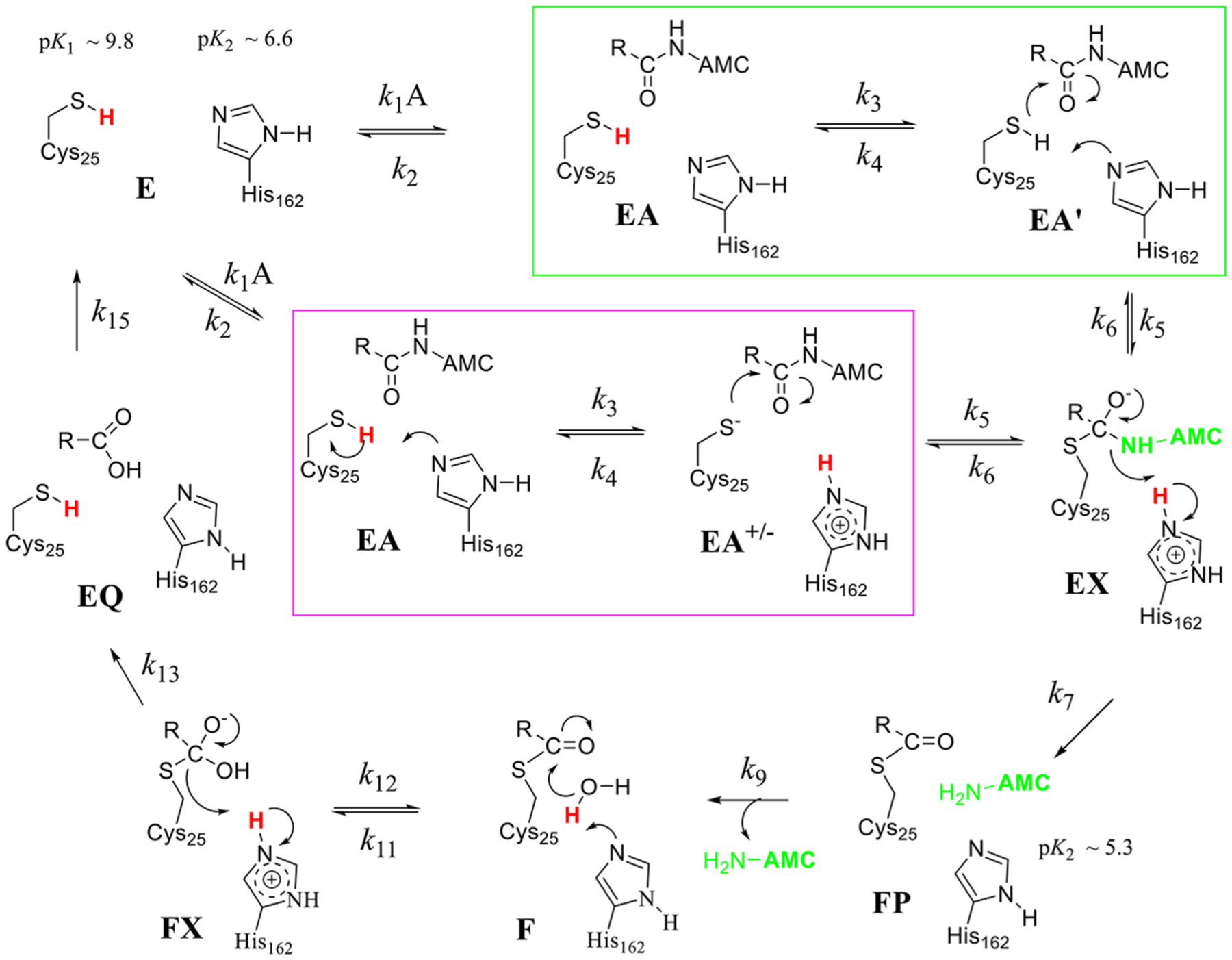
Proposed catalytic mechanisms for cruzain each involving acylation and deacylation half-reactions. Ligand-free cruzain contains neutral forms of and that are not positioned for proton transfer until substrate is bound. In mechanism 1 (green box), proton transfer from to is concerted with thiolate attack on the amide carbonyl to form tetrahedral intermediate EX. This occurs in two discrete steps in mechanism 2 (magenta box) in which the thiolate of attacks the amide. Steps in the deacylation half-reaction are the same for both mechanisms: thio-ester form undergoes attack by a hydroxide ion to form a second tetrahedral intermediate FX, which collapses to form the carboxylate product. Protons involved in primary solvent kinetic isotope effects are colored red. Free AMC is colored light green where it is expected to show full fluorescence.
