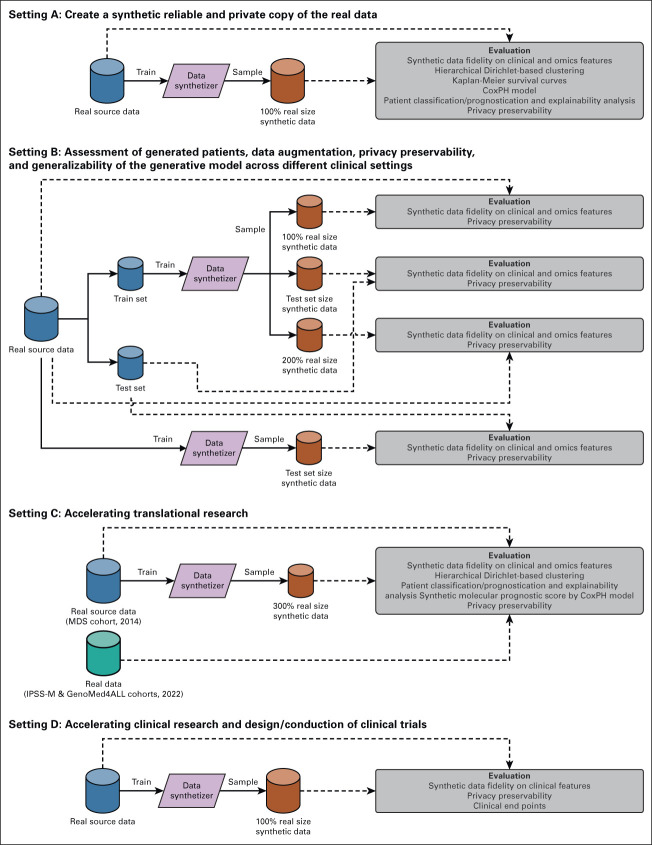
FIG 1.
Overview of experimental settings to validate synthetic data. Setting A: Create a synthetic reliable and private copy of the real data. Setting B: Assessment of generated patients, data augmentation, privacy preservability, and generalizability of the generative model across different clinical settings. Setting C: Accelerating translational research. Setting D: Accelerating clinical research and design/conduction of clinical trials. IPSS-M, Molecular International Prognostic Scoring System; MDS, myelodysplastic syndromes.