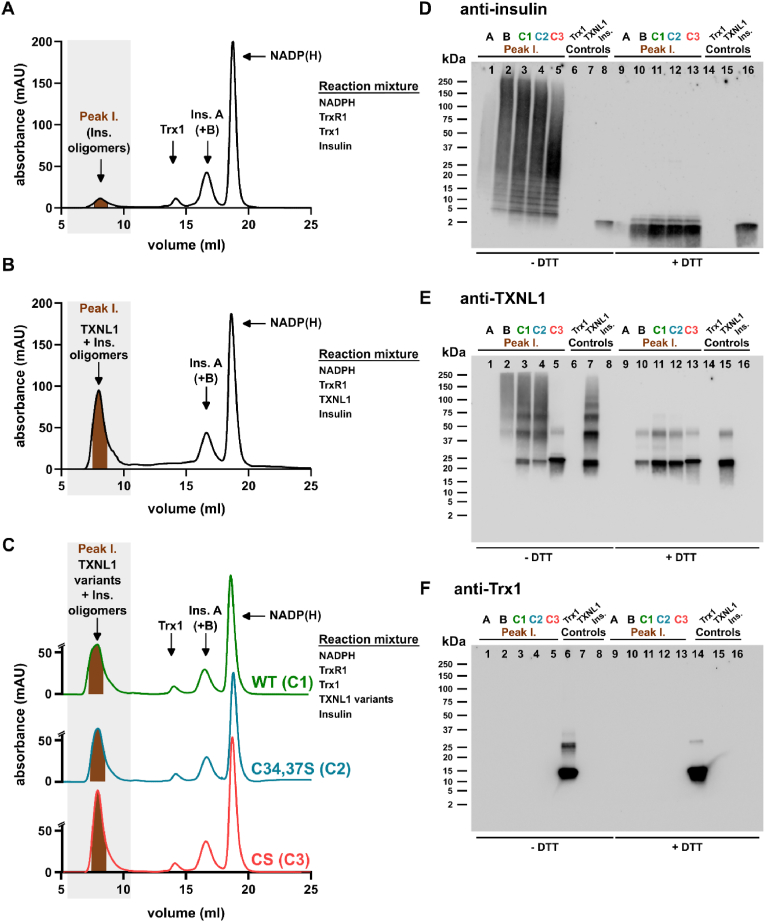
Fig. 8.
Formation of soluble high molecular weight TXNL1-reduced insulin complexes with their isolation by gel filtration do not require the cysteine residues of TXNL1. Here Peak I from the chromatograms of insulin reduction mixtures were analyzed in further detail with the proteins in the different Peak I fractions detected side-by-side in the same Western blots. In (A) the chromatogram of the sole Trx1-catalyzed reaction is shown (same as in Fig. 7A), (B) shows the chromatogram with products of the reaction carried out by Trx1 in the presence of wildtype TXNL1 (same as in Fig. 7E) and in (C) the chromatograms upon addition of the different cysteine mutants of TXNL1 are also shown as overlays for facilitated comparisons, as indicated (the final one same as in Fig. 7I). The chromatogram with addition of wildtype TXNL1 is shown in green and is abbreviated as chromatogram “C1”, that with addition of the active site mutant TXNL1 C34,37S is in blue and named “C2”, and that with addition of the full cystine-to-serine mutant TXNL1 CS is in red and named “C3”. The abbreviations relate to the labeling of the corresponding lanes in the Western blots, where aliquots (2 μl) of the Peak I fractions from the corresponding chromatograms A, B, C1, C2 and C3, were analyzed for presence and migration of (D) insulin B chain, (E) TXNL1, and (F) Trx1, along with control proteins as indicated. All conditions were the same as in Fig. 6. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)