Abstract
Objective
Hypophosphatasia (HPP) is a rare disease characterized by incomplete or defective bone mineralization due to a mutation in the alkaline phosphatase (ALP) gene causing low levels of ALP. Disease presentation is heterogeneous and can present as a chronic pain syndrome like fibromyalgia (FM). Our objective was to determine if there are any potential patients with HPP in the group of patients who were diagnosed with FM. Antiresorptive therapy use can trigger atypical femur fractures in patients with HPP.
Methods
We performed a retrospective chart review of all patients 18 years or older at a single academic center who were diagnosed with FM and had either a low or a normal ALP level. The following characteristics were reviewed: biological sex; age; history of fractures; diagnosis of osteoporosis, osteopenia, osteoarthritis, and chondrocalcinosis; genetic testing; vitamin B6 level testing; and medications.
Results
Six hundred eleven patients with FM were identified. Two hundred had at least one low ALP level, and 57 had at least three consecutively low measurements of ALP, 44% of which had a history of fractures. No patients had vitamin B6 levels checked. None of the patients had previous genetic testing for HPP or underwent testing for zinc or magnesium levels.
Conclusion
The percentage of patients with FM who were found to have consistently low ALP levels was 9.3%. None had vitamin B6 level or genetic testing, suggesting that the diagnosis was not suspected. It is important to diagnose HPP given the availability of enzyme replacement therapy to prevent complications from HPP such as fractures. Our data support screening for this condition as a part of the initial workup of FM.
INTRODUCTION
Hypophosphatasia (HPP) and fibromyalgia (FM) share many clinical features, many of which are nonspecific (Figure 1). FM is common, affecting 1.3% to 8% of the population in the US (1). According to the Tissue Nonspecific Alkaline Phosphatase Gene Mutation Database, the prevalence of the severe form of the disease is 1 in 100,000; however, the less severe forms are reported to be as common as 1 in 6000 (2, 3). HPP has a spectrum of severity and is characterized as a defect in bone mineralization leading to fractures and, perhaps most similarly, muscle pain, chronic pain, and osteoarthropathy (2, 4, 5, 6, 7). There are case reports that have described patients with adult‐onset HPP who were initially misdiagnosed with FM (4, 7, 8). Although FM and HPP may present similarly in their clinical signs and symptoms of chronic musculoskeletal pain, an accurate diagnosis would guide treatment discussion and counseling. Thus, it is essential to be able to identify patients with HPP who may be mistakenly diagnosed with FM. Alkaline phosphatase (ALP) should be normal in patients with FM and low in patients with HPP due to any number of mutations in the ALP gene. A history of premature tooth loss may be present in those with HPP and is not a feature of FM, so a dental history should be obtained in patients with chronic musculoskeletal complaints. If HPP is suspected in a patient with a low ALP level, vitamin B6 levels and phosphoethanolamine (PEA) levels in either urine or serum (which are elevated in HPP) should be checked after alternative reasons for a low ALP level have been ruled out. Genetic testing is desirable but not required for diagnosis (3, 9, 10).
Figure 1.
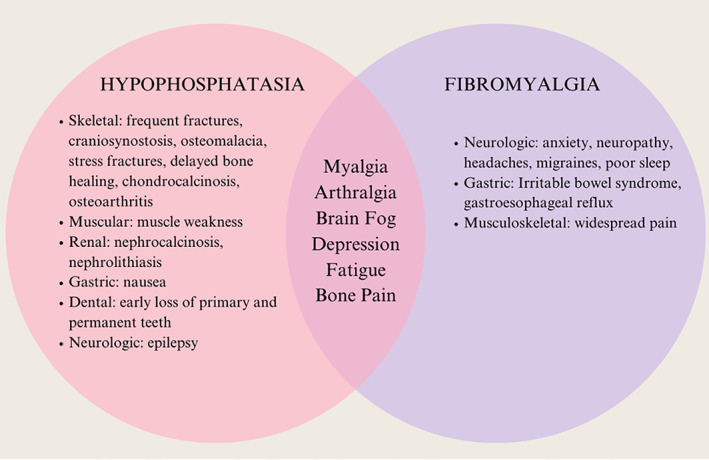
Clinical manifestations of HPP and FM. FM, fibromyalgia; HPP, hypophosphatasia. Sources: Galvez‐Sánchez CM, Reyes del Paso GA. Diagnostic criteria for fibromyalgia: critical review and future perspectives. J Clin Med 2020;9:1219. Schmidt T, et al. Prevalence of low alkaline phosphatase activity in laboratory assessment: is hypophosphatasia an underdiagnosed disease? Orphanet J Rare Dis 2020;16:1–8.
Due to the heterogeneity of presentation of clinical signs and symptoms, lack of awareness, and overlap of presentation with common conditions, HPP is often underdiagnosed or misdiagnosed (11, 12, 13). The identification of patients with HPP is important because HPP may result in an increased incidence of atypical femur fractures. The role of antiresorptive medications in these patients is unclear, and some argue that bisphosphonates are contraindicated in this population. However, enzyme replacement therapy is available, which reduces the risk of fractures in these patients, making identification of these patients among the general osteoporosis population crucial (14, 15). Enzyme replacement therapy, asofotase alfa, is used in patients with HPP to prevent fractures in addition to treating the signs and symptoms of the disease. This medication has been shown to improve many outcomes in HPP, including 6‐minute walk tests, motor function, muscle strength, and functional status. However, this would not provide any benefits to patients with FM (16). Other causes of low ALP level, such as hypomagnesemia, zinc deficiency thyroid disease, use of bisphosphonate therapy (which causes mildly low ALP levels), and Wilson's disease, should be ruled out in this patient population.
ALP has been shown to be a robust biomarker for detecting ALPL variants leading to clinical HPP. Tornero et al evaluated ALPL variants based on ALP measurements. Patients with persistently low ALP levels, defined as at least two measurements less than or equal to 35 IU/L and no measurements greater than 45 IU/L, had the highest specificity (97.8%) for having a pathogenic variant of the ALPL gene (10). In addition, patients presenting with a genetic variant of the ALPL gene had a statistically significant clinical association with musculoskeletal pain. Thus, they have suggested that consecutively low ALP may be a surrogate serological marker for HPP. To determine whether patients diagnosed with FM could actually have HPP at our institution, we performed a retrospective chart review and used ALP as a biomarker to detect potentially undiagnosed HPP.
PATIENTS AND METHODS
This study was approved by the local Institutional Review Board (IRB). We performed a retrospective chart review of all patients 18 years or older at a single academic center who were diagnosed with FM based on International Classification of Diseases (ICD) codes for FM (Tenth Revision [ICD10] code M79.7 or Ninth Revision [ICD9] code 729.1) between May 2015 and March 2022. The patients were included in the study if they had either low or normal ALP levels based on the reference range for our home institution's laboratory (37‐132 U/L). Those with any elevated ALP readings were excluded from this study. The number of consecutively low ALP levels were noted. Biologic sex, age, history of fracture, bisphosphonate use, and a diagnosis of osteoporosis, osteopenia, chondrocalcinosis, osteoarthritis, malnutrition, and thyroid disease were noted. The use of other antiresorptive therapy such as denosumab were not recorded for this study. Other liver function testing was not noted. In patients with three or more low ALP readings (below the reference range of 37 U/L), charts were reviewed for evidence of HPP testing, such as genetic testing and orders for vitamin B6 level. For patients with three or more low ALP readings, charts were reviewed by three investigators. For patients with normal ALP levels, the Epic Slicer Dicer tool was used to obtain demographics and clinical data and was used to perform descriptive statistics (Verona, WI). Patients were thought to potentially have undiagnosed HPP if there were three or more consecutive ALP readings below 37 U/L. This cutoff was selected because this is the level used at our institution. Patient data were deidentified in accordance with IRB approval. Patient race and ethnicity were not recorded for this study. χ2 tests were performed using GraphPad Prism 9 (San Diego, CA).
RESULTS
Six hundred eleven patients were identified to have been diagnosed with FM by ICD10 or ICD9 coding during our study period, with no ALP readings above the normal reference range. Four hundred eleven patients were found to have ALP levels consistently within normal range during the time of the study. One hundred forty‐three patients had one or more nonconsecutively low ALP level(s). Fifty‐seven patients were found to have three consecutively low ALP readings (Table 1). Of patients with any low ALP level documented, none of the patients had an order for vitamin B6 testing or genetic testing. The average age of the patients was not found to be statistically significantly different (P = 0.4819) between groups. The proportion of male patients was higher in the group with three consecutively low ALP readings and the group with one or more nonconsecutively low ALP readings when compared with the normal‐ALP group (17% and 20% vs. 3%, P < 0.05). None of the patients carried a diagnosis of HPP, malnutrition, thyroid disease, or Wilson's disease.
Table 1.
Clinical differences between fibromyalgia groups
| Three consecutive low ALP readings (<37 U/L; n = 57) | One or more nonconsecutive low ALP readings (<37 U/L; n = 143) | Normal ALP (37‐132 U/L; n = 411) | P value | |
|---|---|---|---|---|
| Age (y) | 52 (±17.8) | 52 (±18.6) | 54.5 (±16.4) | 0.4819 |
| Female participants, % | 83% (n = 47) | 80.0% (n = 115) | 97.0% (n = 399) | <0.0001 |
| History of fracture, % | 44% (n = 25) | 33% (n = 48) | 11% (n = 46) | <0.0001 |
| Osteoporosis, % | 44% (n = 25) | 22% (n = 32) | 12% (n = 49) | <0.0001 |
| Osteopenia, % | 4.% (n = 6) | 7.7% (n = 11) | 16% (n = 67) | <0.0276 |
| Chondrocalcinosis, % | 2% (n = 1) | 0% (n = 0) | 4.3% (n = 18) | n/a |
| Osteoarthritis, % | 49% (n = 28) | 24% (n = 35) | 37.2% (n = 153) | <0.0017 |
Abbreviations: ALP, alkaline phosphatase; n/a, not applicable.
The rate of fracture was higher in the group with three consecutively low ALP readings compared with the normal‐ALP group (44% vs. 11%, P < 0.05). Other differences between groups, such as presence of comorbid osteoporosis, osteopenia, chondrocalcinosis, and osteoarthritis, were investigated. Except for chondrocalcinosis, all were present in higher frequency in the low‐ALP group although the low absolute numbers make causation impossible to comment on as a clinical utility of potential markers of undiagnosed HPP. These differences are highlighted as clinical trends, but this study was not powered to be able to distinguish between groups for this purpose. Of the patients with ALP scores of 37 or less on three consecutive occasions, 44% had a history of fractures (n = 25), 4% had been diagnosed with osteopenia by Dual‐energy X‐ray absorptiometry (DXA) scan (n = 6), and 44% were diagnosed with osteoporosis (n = 25), either by fragility fracture or DXA. Twenty‐eight percent of patients in this group were taking bisphosphonates (n = 16). A limitation of this study is the inability to determine whether the low ALP levels in these 16 patients were present prior to the administration of bisphosphonates due to clinical documentation in the medical record and the research design. This is a significant clinical concern and should be investigated separately. Due to the deidentified nature of the study and the lack of confirmatory testing for HPP, these patients were not contacted regarding this potential interaction. Further investigation is warranted in this patient population.
DISCUSSION
To date, there have been no studies investigating ALP levels in patients with FM. However, there have been studies showcasing adult‐onset HPP that identified patients with a previous diagnosis of FM (2, 5). There have been several case reports of patients initially diagnosed with FM who were later found to have adult‐onset HPP (5, 8, 16). One study noted that 13 adults with HPP were previously diagnosed with FM prior to genetic testing (2). One study reported that consistently low ALP levels can have as high as 98% specificity in the diagnosis of HPP (10). However, across all studies it is noted that a definitive diagnosis of HPP should be made based on the combination of consistently low ALP levels in the absence of another etiology, clinical signs and symptoms of HPP, and elevated vitamin B6 or PEA level. Low ALP levels can serve as an initial screening tool for patients with musculoskeletal pain and should trigger further work‐up (2, 15). The biggest limitation of our study is the low number of patients with three consecutively low readings of ALP below reference range. Another large limitation in this study is the retrospective nature of investigation and lack of ability to genetically test patients thought to be potentially suffering from HPP.
A limitation of this study is the small number of patients in the group (n = 57) that had three consecutively low ALP readings, 9.3% of our patient cohort. It is possible that these patients may be suffering from HPP but, due to lack of awareness and understanding of the disease, were misdiagnosed as having FM. This also suggests the possibility that current epidemiologic knowledge of the milder form of HPP may be an underestimation of the true prevalence of disease. These results exemplify the importance of serial ALP level measurements and the inclusion of bone and dental health history in clinical work‐up of musculoskeletal pain. One limitation in this study is the lack of available dental history due to the retrospective nature of the study and the inability to access dental records within the electronic health record. In our study, there were more men in the group thought to potentially be those with undiagnosed HPP. Men have lower bone turnover, which may explain the gender differences found in this study. Furthermore, postmenopausal female patients have higher normal values of ALP, which may obscure the diagnosis of this patient population. In Berkseth's characterization of a large HPP population, approximately 31% were men (11). The group most aligned with a potential diagnosis of HPP, those with three or more consecutively low ALP readings, was found to be the group with the highest percentage of male patients at 17%. Biological sex differences in pathology and clinical presentation are topics that should be researched in the future.
Furthermore, if this investigation is not completed as part of osteoporosis secondary causes work‐up, HPP may go undiagnosed. The availability of asofotase alfa to treat HPP should prompt clinicians to uncover these patients in their osteoporosis patient panel. The goal of this study was not to investigate this question; however, this important work should be undertaken. Patients with HPP may develop atypical femur fractures as part of their disease, which are commonly treated with antiresorptive antiosteoporotic therapies. Some argue that this class of drugs are contraindicated in the treatment of HPP and should be avoided (15). Although this class of drugs can decrease the ALP, it is not typical that the level goes below the reference range. For this reason, we did not eliminate from our study the patients who were taking these medicines.
Although low levels of ALP are suggestive of HPP, there are other multiple causes of low ALP levels. Some alternative causes of low ALP include hypothyroidism, malnutrition, cardiac surgery, cardiopulmonary bypass, and magnesium deficiency (17). To complicate matters further, anemia has also been reported to cause low ALP levels; however, there are data to suggest that it may also be part of the spectrum of HPP (15, 17, 18). Other conditions include celiac disease, Wilson's disease, massive transfusion of blood or plasma, early pregnancy, and drugs such as oral contraceptives (15). Conversely, autoimmune diseases have been shown to increase ALP levels (19). The effect of malignancy on ALP levels is not known. However, those malignancies with metastasis to bones can also increase the ALP levels (20). To avoid these potential confounders, those with elevated ALP levels were excluded from our study. Only one of our patients had previously undergone cardiac surgery and was on cardiopulmonary bypass. None were reported to have celiac disease or Wilson's disease. There were no reported massive transfusions or pregnancy. However, of 57 patients, 27 did report other conditions such as systemic lupus erythematosus, rheumatoid arthritis, polymyositis, and malignancy including cervical cancer, breast cancer, uterine cancer, and leukemia. Magnesium levels and thyroid stimulating hormone were not routinely checked in our patient population, making drawing a conclusion about these as potential confounders impossible.
The availability of medical treatment for HPP is perhaps the most pressing reason to identify these patients correctly. As Lefever et al have previously noted, there is an enzyme replacement medication available that has been shown to significantly improve quality of life (4, 5). In addition, Weber et al have shown in a survey‐based study that patients diagnosed with HPP reported a high disease burden. Thus, it becomes important to diagnose these patients earlier rather than later to provide the proper treatment as treatments for FM are ineffective in preventing the other manifestations of HPP such as fragility fracture. Lastly, decreasing time to diagnosis may improve the patient experience and decrease the mental stress of trying to find the diagnosis for their long‐experienced pain (5). Due to the potential clinical consequences that stem from the misdiagnosis of HPP, we urge clinicians to consistently screen for HPP. This can be achieved via serial ALP measurement and subsequent metabolic testing with vitamin B6 and PEA as part of urine and/or serum amino acids and genetic testing in appropriate circumstances when considering the diagnosis of FM and as secondary work‐up for osteoporosis.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. All authors had full access to all of the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Downey.
Acquisition of data
Injean, Lee, Downey.
Analysis and interpretation of data
Tan, Downey.
Supporting information
Disclosure Form:
ACKNOWLEDGMENTS
None.
Deidentified data are safeguarded and stored electronically.
Author disclosures are available at https://onlinelibrary.wiley.com/doi/10.1002/acr2.11591.
REFERENCES
- 1. Chinn S, Caldwell W, Gritsenko K. Fibromyalgia pathogenesis and treatment options update. Curr Pain Headache Rep 2016;20:25. [DOI] [PubMed] [Google Scholar]
- 2. Högler W, Langman C, Gomes da Silva H, et al. Diagnostic delay is common among patients with hypophosphatasia: initial findings from a longitudinal, prospective, global registry. BMC Musculoskelet Disord 2019;20:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hérasse M, Spentchian M, Taillandier A, et al. Evidence of a founder effect for the tissue‐nonspecific alkaline phosphatase (TNSALP) gene E174K mutation in hypophosphatasia patients. Eur J Hum Genet 2002;10:666–8. [DOI] [PubMed] [Google Scholar]
- 4. Weber TJ, Sawyer EK, Moseley S, et al. Burden of disease in adult patients with hypophosphatasia: results from two patient‐reported surveys. Metabolism 2016;65:1522–30. [DOI] [PubMed] [Google Scholar]
- 5. Lefever E, Witters P, Gielen E, et al. Hypophosphatasia in adults: clinical spectrum and its association with genetics and metabolic substrates. J Clin Densitom 2020;23:340–8. [DOI] [PubMed] [Google Scholar]
- 6. Alonso N, Larraz‐Prieto B, Berg K, et al. Loss‐of‐function mutations in the ALPL gene presenting with adult onset osteoporosis and low serum concentrations of total alkaline phosphatase. J Bone Miner Res 2020;35:657–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Szabo SM, Tomazos IC, Petryk A, et al. Frequency and age at occurrence of clinical manifestations of disease in patients with hypophosphatasia: a systematic literature review. Orphanet J Rare Dis 2019;14:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Braunstein NA. Multiple fractures, pain, and severe disability in a patient with adult‐onset hypophosphatasia. Bone Rep 2016;4:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mornet E. Hypophosphatasia. Metabolism 2018;82:142–55. [DOI] [PubMed] [Google Scholar]
- 10. Tornero C, Navarro‐Compán V, Tenorio JA, et al. Can we identify individuals with an ALPL variant in adults with persistent hypophosphatasaemia? Orphanet J Rare Dis 2020;15:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berkseth KE, Tebben PJ, Drake MT, et al. Clinical spectrum of hypophosphatasia diagnosed in adults. Bone 2013;54:21–7. [DOI] [PubMed] [Google Scholar]
- 12. İnci A, Ergin FB, Yüce BT, et al. Hypophosphatasia: is it an underdiagnosed disease even by expert physicians? J Bone Miner Metab 2021;39:598–605. [DOI] [PubMed] [Google Scholar]
- 13. Desborough R, Nicklin P, Gossiel F, et al. Clinical and biochemical characteristics of adults with hypophosphatasia attending a metabolic bone clinic. Bone 2021;144:115795. [DOI] [PubMed] [Google Scholar]
- 14. Sutton RA, Mumm S, Coburn SP, et al. “Atypical femoral fractures” during bisphosphonate exposure in adult hypophosphatasia. J Bone Miner Res 2012;27:987–94. [DOI] [PubMed] [Google Scholar]
- 15. Bianchi ML. Hypophosphatasia: an overview of the disease and its treatment. Osteoporos Int 2015;26:2743–57. [DOI] [PubMed] [Google Scholar]
- 16. Martins L, de Almeida AB, Dos Santos EJ, et al. A novel combination of biallelic ALPL mutations associated with adult hypophosphatasia: a phenotype‐genotype association and computational analysis study. Bone 2019;125:128–39. [DOI] [PubMed] [Google Scholar]
- 17. Lum G. Significance of low serum alkaline phosphatase activity in a predominantly adult male population. Clin Chem 1995;41:515–8. [PubMed] [Google Scholar]
- 18. Millán JL, Plotkin H. Hypophosphatasia ‐ pathophysiology and treatment. Actual Osteol 2012;8:164–82. [PMC free article] [PubMed] [Google Scholar]
- 19. Hanna AN, Waldman WJ, Lott JA, et al. Increased alkaline phosphatase isoforms in autoimmune diseases. Clin Chem 1997;43:1357–64. [PubMed] [Google Scholar]
- 20. Karhade AV, Thio QC, Kuverji M, et al. Prognostic value of serum alkaline phosphatase in spinal metastatic disease. Br J Cancer 2019;120:640–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form:


