Abstract
Objective
Lung disease (LD) is an increasingly recognized complication of systemic juvenile idiopathic arthritis (sJIA). As there are no currently available guidelines for pulmonary screening in sJIA, we sought to develop such an algorithm at our institution.
Methods
A multidisciplinary workgroup was convened, including members representing rheumatology, pulmonary, stem cell transplantation, and patient families. The workgroup leaders drafted an initial algorithm based on published literature and experience at our center. A modified Delphi approach was used to achieve agreement through three rounds of anonymous, asynchronous voting and a consensus meeting. Statements approved by the workgroup were rated as appropriate with moderate or high levels of consensus. These statements were organized into the final approved screening algorithm for LD in sJIA.
Results
The workgroup ultimately rated 20 statements as appropriate with a moderate or high level of consensus. The approved algorithm recommends pulmonary screening for newly diagnosed patients with sJIA with clinical features that the workgroup agreed may confer increased risk for LD. These “red flag features” include baseline characteristics (young age of sJIA onset, human leukocyte antigen type, trisomy 21), high disease activity (macrophage activation syndrome [MAS], sJIA‐related ICU admission, elevated MAS biomarkers), respiratory symptoms or abnormal pulmonary examination findings, and features of drug hypersensitivity‐like reactions (eosinophilia, atypical rash, anaphylaxis). The workgroup achieved consensus on the recommended pulmonary work‐up and monitoring guidelines.
Conclusion
We developed a pulmonary screening algorithm for sJIA‐LD through a multidisciplinary consensus‐building process, which will be revised as our understanding of sJIA‐LD continues to evolve.
INTRODUCTION
Systemic juvenile idiopathic arthritis (sJIA) is a condition with autoimmune and autoinflammatory features characterized by fever, rash, arthritis, and uncontrolled systemic inflammation (1, 2, 3). The advent of biologic disease modifying antirheumatic drugs (bDMARDs) targeting interleukin (IL)‐1 and IL‐6 has greatly improved outcomes for children with sJIA (1, 2, 3, 4, 5, 6). Although IL‐1 and IL‐6 inhibitors are now considered first‐line treatment, widespread use of these medications has coincided temporally with an increased incidence of pulmonary involvement, including pulmonary arterial hypertension and/or interstitial lung disease (LD). Collectively, these pulmonary manifestations are known as sJIA‐LD (7, 8).
sJIA‐LD has several distinctive characteristics, including histopathologic features of pulmonary alveolar proteinosis and/or lipoid pneumonia (7, 8, 9). Chest imaging may show pleural and septal thickening, ground glass opacities, peripheral consolidation, lymphadenopathy, and/or a crazy paving pattern (7, 9). The clinical presentation of sJIA‐LD is variable. Some children have imaging findings of sJIA‐LD without significant respiratory symptoms, whereas others develop rapidly progressive LD with high fatality (7, 9).
Reports of the prevalence of LD in children with sJIA have ranged between 1.5% and 11%, although rates may be higher as many patients are asymptomatic (10, 11). Similarly, mortality rates are difficult to determine. Over half of the patients with sJIA‐LD died in the first published cohorts; however, these initial reports likely selected for patients with the most severe forms of the disease (7, 8, 9). The true burden of LD in sJIA is undefined due to the unstandardized approach to pulmonary screening, highlighting the importance of developing consensus around a strategy to identify patients with sJIA‐LD.
To standardize our institution's approach to pulmonary evaluation in sJIA‐LD, we developed an algorithm based on review of the literature and input from key stakeholders. Acknowledging that our understanding of pulmonary screening in sJIA‐LD is limited, we intend to use this consensus guideline as a foundation for future iterative changes as more information on sJIA‐LD becomes available.
PATIENTS AND METHODS
Workgroup leaders
The workgroup leaders were two rheumatologists (JCC, LAH), a rheumatology fellow (HW), and a pulmonologist (AC), all with expertise in sJIA.
Workgroup
Rheumatology attending physicians and rheumatology fellows‐in‐training at our institution were invited to participate. Pulmonary attending physicians and pulmonary fellows with training in inflammatory LDs were approached to join the workgroup. A stem cell transplant attending physician who has treated patients with sJIA with LD, as well as the parents of a patient with sJIA‐LD, were also asked to be workgroup members (Supplementary Figure 1). A modified Delphi approach was used to achieve consensus that involved three rounds of anonymous voting and an in‐person conference to facilitate discussion (Figure 1) (12). All workgroup members contributed to at least one round of voting.
Figure 1.
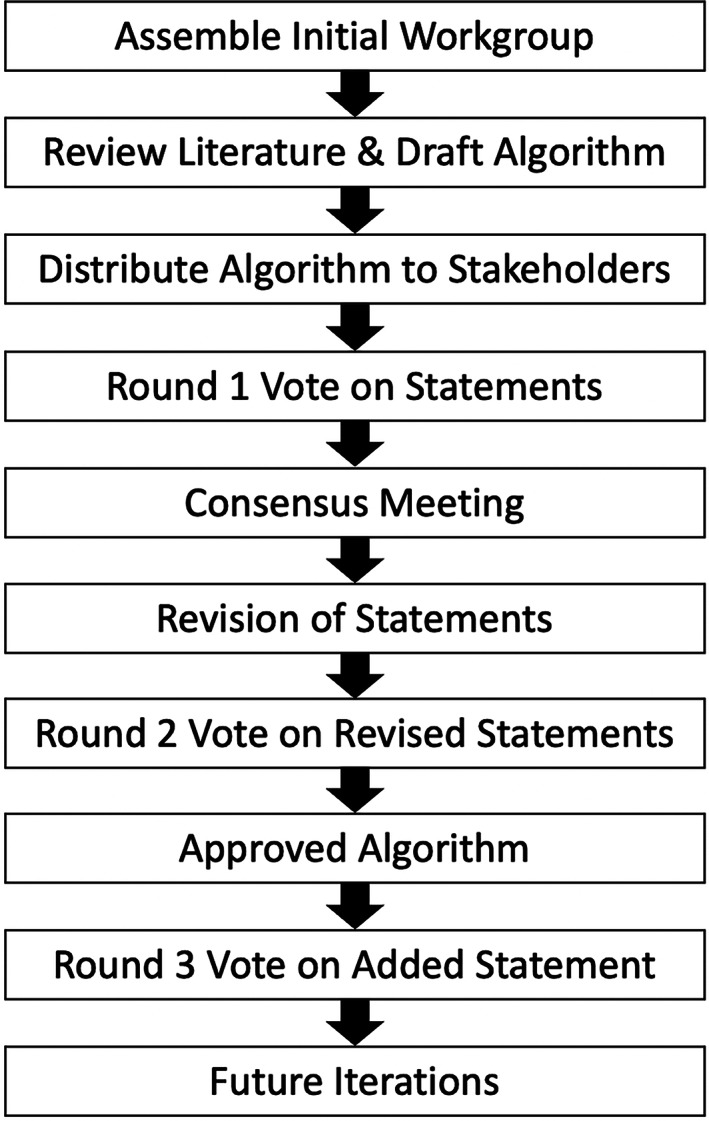
Process used for algorithm development. The systemic juvenile idiopathic arthritis lung disease screening algorithm was developed based on a modified Delphi approach by which statements were voted upon, discussed, refined, and reevaluated by a collaborative workgroup containing diverse stakeholders. Statements rated as appropriate with a moderate or high level of consensus were organized into the approved version of the algorithm.
Evidence review
During July and August 2022, workgroup leaders reviewed relevant publications in PubMed, published abstracts, and conference proceedings. The initial draft of the screening algorithm was developed based on this literature review, expert opinion, and experience at our center. This algorithm was divided into individual statements for voting.
Round 1 voting
The initial draft of the screening algorithm and accompanying voting statements were circulated to the workgroup prior to the consensus meeting. The Excel‐based survey allowed participants to vote asynchronously and anonymously using the RAND/University of California at Los Angeles Appropriateness Method (13). Participants voted on the appropriateness of each statement using the following numeric system: one to three disagree, four to six uncertain, and seven to nine agree. Statements with a median score of greater than or equal to seven were defined as appropriate. High consensus was achieved if 100% of votes were in the same tertile. Lack of consensus was defined as having more than four votes in each of the lower and upper tertiles. All other scenarios were considered moderate levels of consensus. Completed surveys were sent to a nonvoting research assistant who collated and deidentified the responses.
Consensus meeting
Participants attended the consensus meeting in‐person or by video conference. The discussion focused on statements that were rated as inappropriate/uncertain or lacked a high degree of consensus.
Round 2 voting
After the consensus meeting, workgroup leaders revised the voting statements and screening algorithm. The workgroup members rated the revised statements in the same manner as the first round of voting. Approved statements with moderate‐to‐high‐consensus were incorporated into the algorithm.
Round 3 voting
The workgroup voted on one additional statement that was added after piloting use of the algorithm.
RESULTS
Consensus process used to develop the sJIA‐LD screening algorithm
The overarching objective was to develop a screening algorithm for sJIA‐LD with the target audience being pediatric rheumatologists and pulmonologists who care for children with sJIA. During the first round of voting, workgroup members rated the appropriateness of 20 statements that were organized into the initial draft of the sJIA‐LD screening algorithm (Supplementary Table 1). Of these 20 statements, all but 2 were rated as appropriate with a moderate or high degree of consensus (Supplementary Table 1). A second round of voting took place on 10 statements that were modified based on discussion at the meeting, all of which were rated as appropriate with moderate‐to‐high levels of consensus (Supplementary Table 2). One statement that continued to have five votes in the uncertain range during the second round of voting was ultimately excluded by the workgroup leaders because of significant concerns expressed during the consensus meeting. The approved statements were incorporated into the final sJIA‐LD screening algorithm (Figure 2).
Figure 2.
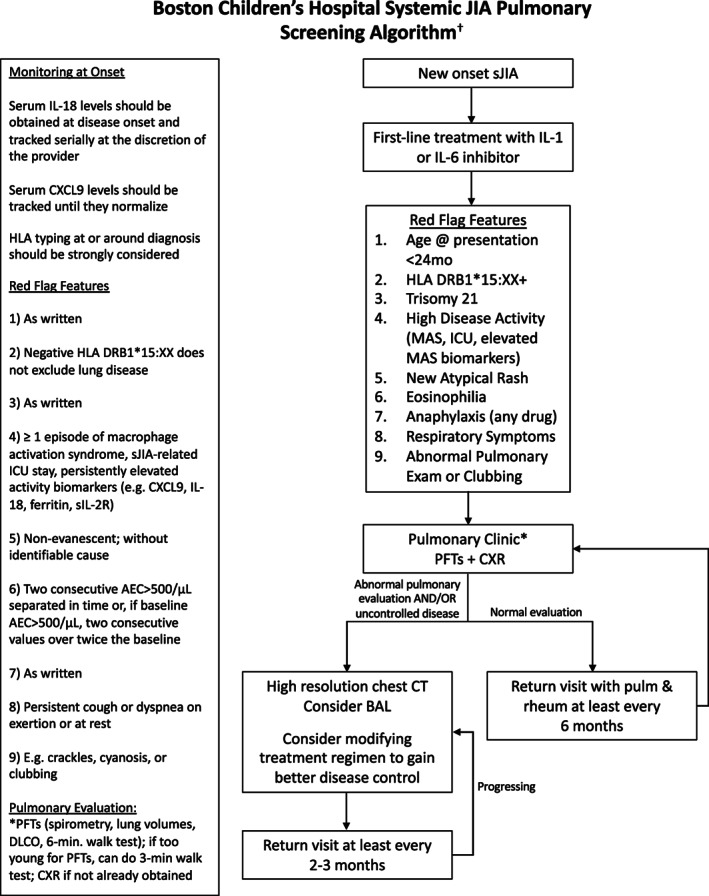
Pulmonary screening algorithm for lung disease in patients with sJIA. The approved algorithm outlines a consensus‐based approach to pulmonary screening for LD in patients with sJIA at our center. Patients with newly diagnosed sJIA and at least one red flag feature are referred for pulmonary evaluation and follow up. †This algorithm was developed for educational purposes only and for use in the Rheumatology and Pulmonary Programs at Boston Children's Hospital. Decisions about evaluation and treatment are the responsibility of the treating clinician and should always be tailored to individual clinical circumstances. AEC, absolute eosinophil count; BAL, bronchoalveolar lavage; CT, computed tomography; CXR, chest x‐ray; DLCO, diffusing capacity for carbon monoxide; HLA, human leukocyte antigen; IL, interleukin; ICU, intensive care unit; JIA, juvenile idiopathic arthritis; MAS, macrophage activation syndrome; PFT, pulmonary function test; sIL‐2R, soluble IL‐2 receptor; sJIA, systemic juvenile idiopathic arthritis.
Target patient population
The screening algorithm was designed for children with new‐onset sJIA without known LD. Most patients with sJIA at our center receive first‐line treatment with IL‐1 or IL‐6 inhibitors as recommended by existing treatment guidelines (14, 15). Nevertheless, the appropriateness of this practice was approved by the workgroup in the first round of voting, with IL‐1 inhibitors receiving a high level of consensus and IL‐6 inhibitors receiving a moderate level of consensus.
Identification of clinical features that may confer risk for sJIA‐LD
LD in sJIA can be insidious, and affected patients may lack respiratory symptoms despite abnormalities on chest imaging (7, 8, 9, 16). The subtle presentation of sJIA‐LD is further complicated by onset at a young age (<2 years of age) when children are less likely to report typical symptoms and are unable to perform standard pulmonary function tests (PFTs) (7). Therefore, the workgroup sought to gain consensus on “red flag” clinical characteristics that may confer risk for sJIA‐LD and be used to identify a population of patients for pulmonary screening. The sJIA‐LD screening algorithm was constructed so that the presence of any red flag feature would result in referral for a pulmonary evaluation.
Baseline patient characteristics
Several studies identified an association between the early age of sJIA disease onset and sJIA‐LD (7, 9). In a multicenter retrospective cohort, the median age of disease onset in patients with sJIA with LD was 2.8 years versus 5.2 years in the comparator group in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry (9). Similarly, Schulert et al found that patients with sJIA with LD at their center were more likely to be diagnosed at 2 years of age or younger compared with children without sJIA‐LD (7). The workgroup achieved a high level of consensus on including the age of less than 24 months at disease presentation as a red flag feature.
HLADRB1*15 alleles have been associated with delayed type drug hypersensitivity‐like reactions (DTR; eosinophilia, atypical rash, transaminitis) in patients with sJIA treated with bDMARDs (17); however, the relationship between HLADRB1*15 alleles and sJIA‐LD is unclear. In a retrospective review of patients with sJIA at our center, these alleles were common (present in 49% of children with available human leukocyte antigen [HLA] typing) and more frequent in individuals who presented with MAS before treatment was initiated compared with those without this genotype (11). These findings in our cohort suggest that HLADRB1*15 alleles may be associated with greater disease severity in sJIA. During the consensus conference, there was extensive discussion on the utility of HLA typing patients with sJIA and including HLADRB1*15 alleles as a red flag feature. There was concern about the high incidence of this allele in our patient population, which has also been observed in other cohorts (11, 18). In addition, uncovering HLA‐DRB1*15 positivity in children without symptoms of DTR or LD could cause inadvertent harm, including unnecessary diagnostic intervention and anxiety for patients and families, especially given uncertainty regarding its significance in relation to LD. Regardless, after discussion, there was a high level of consensus that these HLA alleles may be an important prognostic factor and should inform decisions about pulmonary screening. There was also perceived value in collecting this information systematically to inform future research and quality improvement efforts. We thus recommended universal HLA typing for all patients at sJIA diagnosis and referring patients with DRB1*15 positivity for pulmonary evaluation.
A diagnosis of trisomy 21 was included as a final high‐risk baseline feature after the sJIA‐LD screening algorithm had been piloted for a few months, given the reported association with sJIA‐LD (9).
High sJIA disease activity
Previous studies have demonstrated an association between MAS in sJIA and LD (7, 8, 19, 20). Our retrospective cohort study also found that a high proportion of patients with sJIA with LD had features associated with severe disease, such as history of MAS or a sJIA‐related ICU admission prior to diagnosis of LD (11). Clinically available biomarkers for MAS in patients with sJIA include soluble IL‐2 receptor, ferritin, IL‐18, and CXCL9 (21, 22, 23, 24, 25, 26). Further, patients with sJIA‐LD have significantly higher IL‐18 levels compared with patients with sJIA without LD (7). Based on prior literature and experience at our center, the panel reached consensus that features of high sJIA disease activity should prompt referral for pulmonary screening, including prior MAS, history of ICU admission for sJIA, and persistently elevated MAS biomarkers. The algorithm recommends baseline and routine monitoring of IL‐18, CXCL9, and other laboratory parameters indicative of active disease.
Drug hypersensitivity‐like symptoms
Eosinophilia and nonevanescent rash have been reported in patients with sJIA with LD (17). The underlying pathophysiology that drives these symptoms is unclear with hypotheses ranging from a DTR‐like response to bDMARDs to Th2 polarization in susceptible patients because of environmental exposures (17, 27). At our center, eosinophilia in patients with sJIA was associated with increased rates of pretreatment MAS, suggesting it may be a marker of increased sJIA disease activity (11). After discussion at the consensus meeting, the workgroup reached moderate consensus that these features should lead to pulmonary screening.
Immunoglobulin E–mediated drug reactions, such as anaphylaxis to tocilizumab, have also been reported in patients with sJIA with LD (7, 9). In the first round of voting, the workgroup rated a statement on whether a history of hives or anaphylaxis to any medication should be considered a red flag feature as uncertain. After the consensus meeting, only a history of anaphylaxis to any medication was included in the final pathway.
Pulmonary features
There was high consensus that respiratory symptoms (cough, dyspnea) or physical examination features of pulmonary disease (clubbing, abnormal findings on lung auscultation, evidence of hypoxia) warranted referral for pulmonary evaluation.
Pulmonary evaluation
In the algorithm, patients with at least one red flag feature are referred for pulmonary screening, which initially entails PFTs, a chest x‐ray (CXR), and a pulmonary clinic visit. Ideally, PFTs should include spirometry, lung volumes, diffusing capacity for carbon monoxide (DLCO), and a 6‐minute walk test. For patients too young to complete PFTs, a 3‐ or 6‐minute walk test with continuous oximetry is recommended.
During the first round of voting, the workgroup reached high consensus that an abnormal initial pulmonary evaluation (PFTs, CXR, pulmonary clinic visit) and/or uncontrolled disease activity should prompt further imaging with high‐resolution chest computed tomography (CT). As lower respiratory tract infections may contribute to abnormal pulmonary findings, consideration should also be given to obtaining a flexible bronchoscopy with bronchoalveolar lavage to facilitate diagnostic accuracy.
Because some children with sJIA‐LD have subclinical pulmonary involvement, there was less certainty about whether children with high‐risk features and a normal initial pulmonary evaluation should have a chest CT. This topic was discussed at the consensus meeting. There was concern that high‐quality imaging often requires sedation in young children, exposes them to radiation, and is costly. Ultimately, a high degree of consensus was reached in the second round of voting that children with a normal initial pulmonary evaluation should be followed closely by a pulmonologist, and additional imaging can be obtained at the discretion of the clinical team. The workgroup agreed that there should be a low threshold for chest CT in children with a high burden of sJIA disease activity, even those without respiratory symptoms or abnormal pulmonary testing.
Treatment intensification
Given the association between severe manifestations of sJIA (MAS, ICU level care) and LD, the workgroup reached a high level of consensus that therapy should be intensified in patients with sJIA with uncontrolled disease and/or an abnormal pulmonary evaluation (7, 11). As there are no high‐quality studies of sJIA‐LD that show benefit of a specific treatment, it was left to the treating provider to determine the therapeutic approach, but adequate control of underlying sJIA disease activity was recommended as essential.
DISCUSSION
We used a consensus‐based approach to develop a screening algorithm for LD in children with sJIA. Because there are no existing guidelines on this topic or studies evaluating pulmonary screening in sJIA, our approach relied on literature review, expert opinion, and several rounds of consensus voting by key stakeholders representing rheumatology, pulmonology, stem cell transplantation, and patients/families. As children with sJIA‐LD can be asymptomatic, we sought to identify clinical characteristics that may confer risk for LD to identify pulmonary complications earlier in the disease course when treatment interventions may be more successful.
A limitation of our approach is that clinical characteristics that reliably identify patients at risk for sJIA‐LD are poorly understood. The red flag features that prompt pulmonary evaluation in our algorithm may not have a high sensitivity and specificity for future development of sJIA‐LD. Our algorithm is intended to serve as an initial scaffold upon which iterative changes can be made as the pathogenesis and natural history of sJIA‐LD are better elucidated. As our algorithm may increase the number of referrals to pulmonary clinic, a strong rheumatology–pulmonary collaboration (28) was needed to ensure our pulmonary colleagues agreed with this approach. This algorithm may be less easily implemented in other settings, and local adaptations would need to consider access to testing and subspecialty consultations. Lastly, we acknowledge that systematically screening for sJIA‐LD may increase anxiety among patients and their families. However, the benefits of early identification of LD were deemed to outweigh potential harm, and vigilance may also provide reassurance that sJIA‐LD is either absent or addressed early in the disease course.
The rapid emergence of sJIA‐LD coupled with the lack of clarity on its prevalence and etiology has left clinicians with uncertainty about how to manage children with sJIA. Ongoing efforts to better characterize sJIA‐LD in prospective multicenter cohorts, such as the CARRA registry, are needed. Biomarkers that accurately identify patients at risk for sJIA‐LD will be beneficial and may be forthcoming (29). Novel imaging modalities, such as lung ultrasounds, may be less invasive and reduce radiation exposure but require validation (30). Although these future research directions are promising, clinicians must confront the present conundrum of sJIA‐LD with limited information to guide practice. Our center chose to approach this challenge through multidisciplinary consensus building to develop a systematic screening algorithm for pulmonary complications in patients with sJIA.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Drs. Wobma and Henderson had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Wobma, Casey, Joyce C. Chang, Henderson.
Acquisition of data
Wobma, Bachrach, Farrell, Margaret H. Chang, Day‐Lewis, Dedeoglu, Fishman, Halyabar, Ibanez, Kim, Klouda, Krone, Lee, Lo, Meidan, Prockop, Samad, Son, Nigrovic, Casey, Joyce C. Chang, Henderson.
Analysis and interpretation of data
Wobma, Harris, McBrearty, Henderson.
Supporting information
Disclosure Form:
Appendix S1: Supporting Information
Dr. Womba's work was supported by the National Institute of Allergy and Infectious Diseases, NIH (grant T32‐AI‐007512‐36). Dr. Son has received salary support from Childhood Arthritis and Rheumatology Research Alliance, royalties from Up To Date, and funding from the Centers for Disease Control and Prevention. Dr. Nigrovic's work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH (grant P30‐AR‐070253). Dr. Chang's work was supported by the National Heart, Lung, and Blood Institute, NIH (grant K23‐HL‐148539). Dr. Henderson's work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH (grant K08‐AR‐073339), the National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH (grant P30‐AR‐070253), Career Development Bridge Funding from the Rheumatology Research Foundation, and an All Arthritis grant from the Arthritis National Research Foundation.
Author disclosures are available at https://onlinelibrary.wiley.com/doi/10.1002/acr2.11600.
REFERENCES
- 1. Minoia F, Davì S, Horne A, et al. Clinical features, treatment, and outcome of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a multinational, multicenter study of 362 patients. Arthritis Rheumatol 2014;66:3160–9. [DOI] [PubMed] [Google Scholar]
- 2. Behrens EM, Beukelman T, Gallo L, et al. Evaluation of the presentation of systemic onset juvenile rheumatoid arthritis: data from the Pennsylvania Systemic Onset Juvenile Arthritis Registry (PASOJAR). J Rheumatol 2008;35:343–8. [PubMed] [Google Scholar]
- 3. Petty RE, Southwood TR, Manners P, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004;31:390–2. [PubMed] [Google Scholar]
- 4. Haar NM, Van Dijkhuizen EH, Swart JF, et al. Treatment to target using recombinant interleukin‐1 receptor antagonist as first‐line monotherapy in new‐onset systemic juvenile idiopathic arthritis: results from a five‐year follow‐up study. Arthritis Rheumatol 2019;71:1163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vastert SJ, De Jager W, Noordman BJ, et al. Effectiveness of first‐line treatment with recombinant interleukin‐1 receptor antagonist in steroid‐naive patients with new‐onset systemic juvenile idiopathic arthritis: results of a prospective cohort study. Arthritis Rheumatol 2014;66:1034–43. [DOI] [PubMed] [Google Scholar]
- 6. Yokota S, Imagawa T, Mori M, et al. Efficacy and safety of tocilizumab in patients with systemic‐onset juvenile idiopathic arthritis: a randomised, double‐blind, placebo‐controlled, withdrawal phase III trial. Lancet 2008;371:998–1006. [DOI] [PubMed] [Google Scholar]
- 7. Schulert GS, Yasin S, Carey B, et al. Systemic juvenile idiopathic arthritis–associated lung disease: characterization and risk factors. Arthritis Rheumatol 2019;71:1943–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kimura Y, Weiss JE, Haroldson KL, et al. Pulmonary hypertension and other potentially fatal pulmonary complications in systemic juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2013;65:745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saper VE, Chen G, Deutsch GH, et al. Emergent high fatality lung disease in systemic juvenile arthritis. Ann Rheum Dis 2019;78:1722–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Erkens R, Sinha R, Pickering A, et al. First line treatment using recombinant IL‐1 receptor antagonist in new onset systemic juvenile idiopathic arthritis is an effective treatment strategy, irrespective of HLA DRB1 background [abstract]. Arthritis Rheumatol 2022;74:4555–7. [DOI] [PubMed] [Google Scholar]
- 11. Wobma H, Arvila SR, Taylor ML, et al. Incidence and risk factors for eosinophilia and lung disease in biologic‐exposed children with systemic juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2023. E‐pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dalkey NC. An experimental study of group opinion: The Delphi Method. Futures 1969;1:408–26. [Google Scholar]
- 13. Fitch K. The Rand/UCLA appropriateness method user's manual. Santa Monica: Rand; 2001. [Google Scholar]
- 14. Onel KB, Horton DB, Lovell DJ, et al. 2021 American College of Rheumatology guideline for the treatment of juvenile idiopathic arthritis: therapeutic approaches for oligoarthritis, temporomandibular joint arthritis, and systemic juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2022;74:521–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DeWitt EM, Kimura Y, Beukelman T, et al. Consensus treatment plans for new‐onset systemic juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2012;64:1001–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Canna SW, Schulert GS, De Jesus A, et al. Proceedings from the 2nd Next Gen Therapies for Systemic Juvenile Idiopathic Arthritis and Macrophage Activation Syndrome symposium held on October 3‐4, 2019. Pediatric Rheumatol 2020;18 Suppl 1:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saper VE, Ombrello MJ, Tremoulet AH, et al. Severe delayed hypersensitivity reactions to IL‐1 and IL‐6 inhibitors link to common HLA‐DRB1*15 alleles. Ann Rheum Dis 2022;81:406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lerman AM, Mahmud SA, Alfath Z, et al. High frequencies of HLA DRB1*15 and eosinophilia among patients with systemic juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2023. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gao DK, Salomonis N, Henderlight M, et al. IFN‐γ is essential for alveolar macrophage‐driven pulmonary inflammation in macrophage activation syndrome. JCI Insight 2021;6:e147593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bracaglia C, Minoia F, Kessel C, et al. Systemic juvenile idiopathic arthritis associated lung disease in Europe [abstract]. Arthritis Rheumatol 2022;74:1696–7. [Google Scholar]
- 21. Shimizu M, Nakagishi Y, Inoue N, et al. Interleukin‐18 for predicting the development of macrophage activation syndrome in systemic juvenile idiopathic arthritis. Clin Immunol 2015;160:277–81. [DOI] [PubMed] [Google Scholar]
- 22. Yasin S, Fall N, Brown RA, et al. IL‐18 as a biomarker linking systemic juvenile idiopathic arthritis and macrophage activation syndrome. Rheumatology (Oxford) 2020;59:361–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takakura M, Shimizu M, Irabu H, et al. Comparison of serum biomarkers for the diagnosis of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Clin Immunol 2019;208:108252. [DOI] [PubMed] [Google Scholar]
- 24. Kessel C, Fall N, Grom A, et al. Definition and validation of serum biomarkers for optimal differentiation of hyperferritinaemic cytokine storm conditions in children: a retrospective cohort study. Lancet Rheumatol 2021;3:e563–73. [DOI] [PubMed] [Google Scholar]
- 25. Bracaglia C, De Graaf K, Marafon DP, et al. Elevated circulating levels of interferon‐γ and interferon‐γ‐induced chemokines characterize patients with macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Ann Rheum Dis 2017;76:166–72. [DOI] [PubMed] [Google Scholar]
- 26. Weiss ES, Girard‐Guyonvarc'h C, Holzinger D, et al. Interleukin‐18 diagnostically distinguishes and pathogenically promotes human and murine macrophage activation syndrome. Blood 2018;131:1442–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Binstadt BA, Nigrovic PA. The conundrum of lung disease and drug hypersensitivity‐like reactions in systemic juvenile idiopathic arthritis. Arthritis Rheumatol 2022;74:1122–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wobma H, Perkins R, Bartnikas L, et al. Genetic diagnosis of immune dysregulation can lead to targeted therapy for interstitial lung disease: a case series and single center approach. Pediatr Pulmonol 2022;57:1577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen G, Deutsch GH, Schulert GS, et al. Identification of distinct inflammatory programs and biomarkers in systemic juvenile idiopathic arthritis and related lung disease by serum proteome analysis. Arthritis Rheumatol 2022;74:1271–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vega‐Fernandez P, Ting TV, Mar DA, et al. Lung ultrasound in children with systemic juvenile idiopathic arthritis associated interstitial lung disease. Arthritis Care Res (Hoboken) 2023;75:983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form:
Appendix S1: Supporting Information


