Abstract
Background and Aims
Breast cancer is a multifactorial malignancy with different clinicopathological and molecular characteristics. It is the most frequent cancer in women in terms of both incidence and mortality. Matrix metallopeptidase 1 or MMP1 is a zinc‐dependent endopeptidase associated with several physiological processes through the modification of the extracellular matrix and tumor microenvironment. However, previous results did not suggest any concluding remarks on the correlation between MMP1 gene polymorphisms and the risk of breast cancer.
Methods
A comprehensive literature search was performed in PubMed database to retrieve relevant articles and extract data from suitable ones. The literature written only in English was selected for this review.
Results
A total of 26 articles were included in the present narrative review. From the available studies, it is observed that MMP1 is upregulated in breast cancer tissues and found to be correlated with metastasis and invasion. The expression of MMP1 gene is mediated by numerous factors, including polymorphisms which act as a potential risk factor for the progression of breast cancer. To establish the correlation between genetic polymorphisms in MMP1 and the risk of breast cancer, several case‐control studies, as well as genetic analyses, have been carried out in different ethnicities. The association of genetic polymorphisms in MMP1 with the risk and survival of breast cancer in different populations has been reviewed in this study. Moreover, the structural domain of MMP1 and the role of MMP1 in breast cancer metastasis and invasion are also discussed which will help to understand the potential impact of MMP1 as a genetic biomarker.
Conclusions
This review provides an overview of the MMP1 gene polymorphisms in breast cancer. However, we recommend future studies concentrating on combined analysis of multiple SNPs, gene‐gene interactions, and analysis of epigenetics, proteomics, and posttranscriptional modifications that will provide the best outcome.
Keywords: association, breast cancer, matrix metalloproteinase 1, MMP1, polymorphism, risk
1. INTRODUCTION
Breast cancer (BC) is the most frequent type of malignancy in females throughout the world and is also the leading reason of mortality among them. 1 Approximately 1.6 million new cases of BC patients are reported every year and the incidence is worryingly increasing during the last few years. Besides, the incidence of mortality due to BC has been the highest among women. According to the report from 2018, almost 626,679 patients (6.6% of all cancer‐associated deaths) died globally because of BC. 2 , 3 In spite of substantial improvements in treatment strategies, BC has been the major cause of mortality in women over the past few decades, making it a global health burden. 4
BC is considered a multifactorial and heterogenous malignancy with different clinicopathological and molecular characteristics. A combined effect of various potential risk factors including genetic, epigenetic, population structure, environmental, and sedentary lifestyle results in BC. 5 , 6 Some common factors are physical inactivity, high‐fat diet, hormonal imbalance, early or late menstrual cycle, late pregnancy, dense breasts, old age, high‐stress level, radiation, or environmental carcinogens. 7 BC is a polygenic malignancy and hereditary BC accounts for around 5%–10% of all diagnosed cases. 8 The microenvironment of breasts consists of an extracellular matrix (ECM) and different stromal cells such as endothelial cells, fibroblasts, immune cells, and adipocytes which play a crucial part in the morphogenesis of mammary duct. 9
Matrix metalloproteinases (MMPs), also called matrixins, are metal‐dependent endopeptidases. 10 MMPs are a family of multigene that commonly associate with diverse physiological and pathological mechanisms in the human body required for development and morphogenesis. 11 Typically, MMPs consist of a secretory amino terminal, a cysteine‐switched latency‐mediating pro‐domain, and a Zn2+‐dependent enzymatic domain. Most of the members of the MMPs family also possess a substrate‐specific hemopexin domain‐containing C‐terminal. 12 , 13 , 14 MMPs play a catalytic role during ECM degradation and remodeling. 15 Besides, the activity of some growth factors, proteases, chemokines, cytokines, ligands, proteases, and receptors are also regulated by them. However, loss of MMPs activities leads to angiogenesis, metastasis, cell adhesion, cell migration, differentiation, proliferation, and inflammation, which ultimately develops cancers. 16 , 17 , 18 , 19 , 20 , 21
Based on the structural domains and specificity to particular substrates, MMPs are broadly classified into five major groups namely, collagenases, stromelysins, matrilysins, gelatinases, and membrane‐associated MMPs. MMP1 is from the collagenases group and is one of the most commonly expressed MMPs. It is responsible for the breakdown of collagen type I, II, and III. 22 , 23 Impaired expression of MMP1 has been reported in multiple cancers including breast, 24 lung, 25 prostate, 26 and colon. 27
The present review focuses on the association of MMP1 gene polymorphisms with BC susceptibility, which has not been comprehensively studied or reviewed before. MMP1 genetic polymorphisms have been implicated in various malignancies according to previous studies, but the findings have been inconclusive. The review aims to shed light on the potential role of MMP1 gene polymorphisms in BC by discussing their association with BC risk. It also delves into the structure of the MMP1 and explores its potential role in the development and progression of BC.
2. STRUCTURAL DOMAINS OF MMP1
Generally, MMPs comprise a propeptide domain of around 80 amino acids, an essential metalloproteinase domain (catalytic domain) of around 170 amino acids, a linker (hinge region) peptide of variable lengths (typically 15–65 amino acids), and a hemopexin (Hpx) domain of about 200 amino acids. 28 , 29 , 30 For the activity of a typical MMP, requires a zinc ion (Zn2+) in the catalytic domain beside the proteolytic activation. 31 The human MMP1 structural domains (Figure 1) are mainly an N‐terminal catalytic domain, a linker peptide region, and a C‐terminal Hpx domain in which the catalytic domain of one monomer connects the Hpx domain of another monomer. 32 , 33
Figure 1.
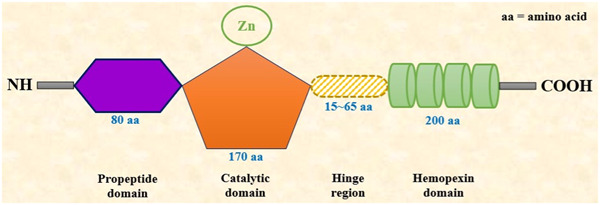
Structural domains of MMP1. MMP, matrix metalloproteinase.
The catalytic domain or metalloproteinase domain of a typical MMP1 contains a conserved sequence of three histidine residues necessary for Zn2+ chelation. The structure of MMP1 catalytic region is almost analogous to other members of MMPs. In length, it is almost 170 amino acid residues long with a catalytic Zn2+ residing in the C‐terminal site. The catalytic domain is connected to the hemopexin domain through a short hinge region. Moreover, the MMP1 metalloproteinase domain carries three calcium‐binding sites in its structure. 31
The catalytic domain of MMP1 is followed by the linker or the hinge region consists of a stretch of about 15–65 amino acid residues. Proline residues commonly construct hinge regions, and the presence of the appropriate hinge structure is vital for the process of collagenolysis. These amino acid residues possess an extensive connection with both the catalytic domain and the Hpx domain of MMP1. This close connection is necessary for the stabilization and the concerted action between the domains in MMP1. Mutations in the hinge region drastically reduce the collagenolytic activity of MMP1 as a result of the movement restrictions between the catalytic and the Hpx domain.
The hemopexin (Hpx) domain of MMP1 begins with Cys259 residue and makes a complete circular structure by connecting to Cys447. The Hpx domain shows the dramatic effect through a significant displacement to the metalloproteinase domain, which widens the cleft located between these domains arranged on the active site face in this enzyme. This attenuated configuration produces residues of the active site as well as the RWTNNFREY (residues 183 to residues 191) segment crucial for collagenolytic activity. The Hpx domain consists of a β‐propeller (4‐bladed) structure and a linking disulfide bond (S‐S) between the first and the fourth blades. Typically, the center of this propeller structure contains a chloride ion and a calcium ion. 21 , 33 This domain is necessary for the interactions between other MMPs. 31
3. SOURCES AND DISTRIBUTION OF MMP1
MMP1 is produced and secreted by several cells in the human body (Table 1). Connective tissues, proinflammatory cells, and different uteroplacental cells such as endothelial cells, platelets, fibroblasts, osteoblasts, chondrocytes, lymphocytes, smooth muscle cells (SMCs), neutrophils, macrophages, cytotrophoblasts, etc. produce and distribute MMP1. MMP1 has a key function in tissue remodeling via increasing the turnover of multiple ECM proteins such as collagens, gelatin, elastin, proteoglycans, and glycoproteins. Collagen and elastin are two prominent proteins necessary for maintaining the vascular wall's structural integrity. MMP1 breaks down collagen substrates I, II, III, IV, VII, VIII, X, and gelatin with variable efficacies. It also breaks down noncollagen ECM substrates including aggrecan, perlecan, versican, proteoglycan link protein, serpins, nidogen, fibronectin, and tenascin. It also degrades casein, antichymotrypsin, IL1β, pro‐tumor necrosis factor‐α, SDF1, antitrypsin, proteinase inhibitor, IGF‐BP3 and 5. 29 , 34 , 35 , 36
Table 1.
Location, tissue distribution and substrates of MMP1 gene.
| Name | Chromosomal location | MW pro/active | Distribution | Collagen substrates | Noncollagen substrates | Other targets and substrates |
|---|---|---|---|---|---|---|
| MMP1 | 11q22.3 | 55/45 KDa | Endothelium, intima, fibroblasts, SMCs, vascular adventitia, platelets, varicose veins | I, II, III, IV, VII, VIII, X, and gelatin | Aggrecan, perlecan, versican, proteoglycan link protein, serpins, nidogen, tenascin | Casein, antichymotrypsin, IL1β, pro‐TNFα, SDF1, antitrypsin, proteinase inhibitor, IGF‐BP3 and 5 |
Abbreviations: IL, interleukin; MMP, matrix metalloproteinase; SMC, smooth muscle cell; TNF, tumor necrosis factor.
4. FUNCTION OF MMP1 IN BC PROGRESSION
Metastasis and invasion of cancer cells happen as a result of several key steps like malignant cell detachment at the main origin, angiogenesis, cellular proliferation, invasion in local regions, intravasation of tumors into the vasculature system, and extravasation of tumors at the distant region. Metastasis and invasion also need different physical barriers, for example, the basement membrane and the surrounding connective tissues. 37 MMP1 is a calcium‐dependent zinc‐containing collagenase that is upregulated in a variety of cancers and involves tumor metastasis and invasion as well as cell proliferation, differentiation, migration, angiogenesis, apoptosis, and immune defense. Moreover, studies showed an inverse correlation between MMP1 overexpression and survival in cancer patients. 38 , 39 , 40
The activity of MMP1 is tightly controlled in normal tissues by proteolytic cleavage, including the negative regulation of TIMPs (tissue inhibitors of metalloproteinases), which is less expressed in malignant tissues. Again, the lower transcription level of MMP1 in normal epithelia is increased in response to various stimuli, for instance, cytokines, chemokines, growth factors, and several hormones. 41 In breast carcinoma, particularly basal‐type cancers, MMP1 is upregulated and shows extensive metastatic properties. It is also associated with progression and relapse‐free survival leading to poor prognosis of BC. There is also a statistically significant difference between stromal cells MMP1 positivity and luminal A, B, and TNBC (triple‐negative BC). Most importantly, MMP1 expression at the BC level carries an independent prognostic value. 42
A recent study by Wang et al. 43 reported that the MMP1 protein expression level is significantly higher (p < 0.05) in TNBC tissues than in estrogen receptor‐positive (ER+) and epidermal growth factor 2 receptor‐positive (EGF2R3+) BC tissues. Moreover, the MMP1 level was significantly elevated in the stromal cells of metastatic lymph node tissues than that of the nonmetastatic tissues in BC. They showed that MMP1 small hairpin RNA in MDA‐MB‐231 and MCF‐7 cells drastically reduced migration, proliferation, and invasion knocking down MMP1 expression, and suggested that MMP1 is differentially regulated in BC.
Another study by Shen et al. 38 demonstrated that upregulated MMP1 leads to the activation of paracrine protease‐activated receptor 1 (PAR1) and promotes growth and distant metastasis of BC tissues. Moreover, the high expression level of MMP1 is correlated with worse survival in all BC patients including ER+ patients. Furthermore, MMP1 increased invasiveness in BC tissues through vascular endothelial growth factor as well as bone morphogenetic protein 2/4. Some recent studies also explicated that MMP1 enhanced tumor cell migration via degrading specific cell adhesion and cell‐matrix adhesion regulatory substrates. This interaction ultimately leads to tumor metastasis and invasion. 44 A previous study by Eiró et al. 45 described that MMP1 expression in host defense cells is linked with the sequential metastasis in the lymphatic system (sentinel lymph nodes, SLN) of BC tissues. An updated analysis by Eiró et al. 46 reported a significant correlation of enhanced MMP1 expression with tumor size and histological grade in BC.
MMP1 upregulation has been identified and confirmed as an important factor for BC metastasis. It has been described that expression of MMP1 is greater in invasive ductal carcinoma (both nonspecific and lymph node metastatic nonspecific) than the normal tissues and lymph node tissues in BC. 47 Cierna et al. 48 also reported that elevated MMP1 expression is correlated with evolution, dissemination, worse prognosis, and shortened survival rate in breast tumor. Moreover, MMP1 induces epithelial to mesenchymal transition promoting the invasiveness of BC cells. The expression of MMP1 in BC samples based on the sample types, individual cancer stages, patient's race, patient's gender, patient's age, BC subclass, menopause status, and nodal metastasis status is depicted in Figure 2A–H. The expression data were retrieved from the publicly available ULCAN database (https://ualcan.path.uab.edu/index.html).
Figure 2.
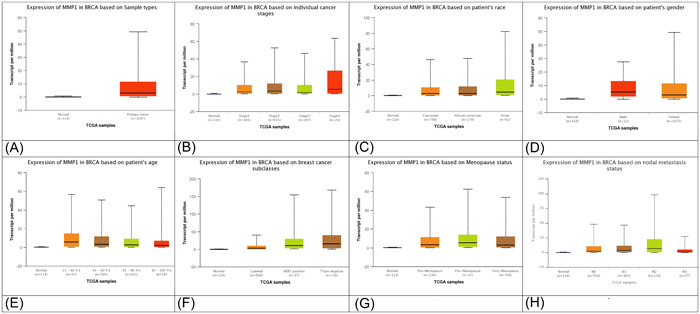
MMP1 expression based on the sample types (A), individual cancer stages (B), patient's race (C), patient's gender (D), patient's age (E), breast cancer subclass (F), menopause status (G), and nodal metastasis status (H). Expression data was retrieved from the publicly available ULCAN database (https://ualcan.path.uab.edu/index.html). MMP, matrix metalloproteinase.
5. ASSOCIATION OF GENETIC POLYMORPHISMS IN MMP1 WITH BC
Almost 90%–95% of BC cases are thought to be sporadic types and result from the combination of both genetic and environmental factors. According to the polygenic models, a combination of numerous low‐risk genes with polymorphisms in the genome sequence can enhance several diseases' vulnerability. 49 The MMP1 expression level might be greatly influenced by polymorphisms, especially single nucleotide polymorphisms (SNPs) that are positioned within or near the promoter site of the MMP1 gene. The association of different genetic polymorphisms of MMP1 has been studied for cancers, especially in BC (Table 2).
Table 2.
MMP1 gene polymorphisms and their association with breast cancer.
| SNPs | Major/minor allele | MAF | Population | Cases/controls | OR | 95% CI | p value | References |
|---|---|---|---|---|---|---|---|---|
| rs5854 | C/T | 0.60 | United States and Mexico | 3592/4183 | 0.82 | 0.69–0.97 | 0.018 | Slattery et al. 22 |
| rs17293823 | G/A | 0.21 | United States and Mexico | 3592/4183 | N/A | N/A | N/A | Slattery et al. 22 |
| rs996999 | C/T | 0.45 | United States and Mexico | 3592/4183 | 1.23 | 1.01–1.50 | 0.039 | Slattery et al. 22 |
| rs17293761 | C/T | 0.15 | United States and Mexico | 3592/4183 | N/A | N/A | N/A | Slattery et al. 22 |
| rs7945189 | C/T | 0.15 | United States and Mexico | 3592/4183 | N/A | N/A | N/A | Slattery et al. 22 |
| rs7125062 | T/C | 0.70 | United States and Mexico | 3592/4183 | 1.15 | 1.01–1.32 | 0.03 | Slattery et al. 22 |
| rs470358 | C/T | 0.84 | United States and Mexico | 3592/4183 | N/A | N/A | N/A | Slattery et al. 22 |
| rs475007 | A/T | 0.90 | United States and Mexico | 3592/4183 | N/A | N/A | N/A | Slattery et al. 22 |
| rs1144393 | T/C | 0.60 | United States and Mexico | 3592/4183 | 0.94 | 0.85–1.04 | 0.03 | Slattery et al. 22 |
| rs1799750 | 1G/2G | 0.49 | Poland | 270/300 | 1.68 | 1.19–2.39 | <0.05 | Przybylowska et al. 50 |
| rs1799750 | 1G/2G | 0.76 | Poland | 299/299 | 1.08 | 0.70–1.67 | >0.05 | Białkowska et al. 16 |
| rs1799750 | 1G/2G | N/A | Sweden | 959/952 | N/A | N/A | >0.05 | Lei et al. 51 |
| rs1799750 | 1G/2G | 0.19 | Italy | 43/164 | 1.51 | 0.65–3.50 | >0.05 | Biondi et al. 52 |
| rs1799750 | 1G/2G | 0.44 | Taiwan | 1232/1232 | 1.03 | 0.91–1.18 | >0.05 | Hsiao et al. 53 |
| rs1799750 | 1G/2G | 0.58 | South India | 300/300 | 2.01 | 1.57–2.59 | <0.05 | Padala et al. 54 |
| rs1799750 | 1G/2G | 0.44 | Taiwan | 1232/1232 | 0.99 | 0.89–1.11 | >0.05 | Su et al. 55 |
| rs1799750 | 1G/2G | 0.53 | Italy | 86/110 | 0.97 | 0.65–1.45 | >0.05 | Ghilardi et al. 56 |
| rs1799750 | 1G/2G | 0.25 | Iran | 100/100 | 0.21 | 0.14–0.33 | <0.05 | Balkhi et al. 7 |
| rs1799750 | 2G/1G | 0.35 | China | 3016/3007 | 1.0 | 0.8–1.30 | 0.45 | Beeghly‐Fadiel et al. 57 |
| rs484915 | A/T | 0.34 | China | 3016/3007 | 1.1 | 0.9–1.50 | 0.76 | Beeghly‐Fadiel et al. 57 |
| rs1155764 | T/G | 0.20 | China | 3016/3007 | 0.8 | 0.5–1.30 | 0.90 | Beeghly‐Fadiel et al. 57 |
| rs509332 | A/G | 0.13 | China | 3016/3007 | 1.4 | 0.8–2.70 | 0.54 | Beeghly‐Fadiel et al. 57 |
| rs470206 | G/A | 0.13 | China | 3016/3007 | 1.3 | 0.7–2.50 | 0.46 | Beeghly‐Fadiel et al. 57 |
| rs2075847 | T/C | 0.24 | China | 3016/3007 | 1.1 | 0.7–1.50 | 0.71 | Beeghly‐Fadiel et al. 57 |
| rs498186 | A/C | 0.46 | China | 3016/3007 | 1.0 | 0.8–1.20 | 0.73 | Beeghly‐Fadiel et al. 57 |
| rs475007 | T/A | 0.36 | China | 3016/3007 | 0.9 | 0.7–1.20 | 0.77 | Beeghly‐Fadiel et al. 57 |
| rs996999 | T/C | 0.49 | China | 3016/3007 | 1.0 | 0.8–1.30 | 0.79 | Beeghly‐Fadiel et al. 57 |
| rs470558 | G/A | 0.11 | China | 3016/3007 | 1.0 | 0.5–2.00 | 0.58 | Beeghly‐Fadiel et al. 57 |
| rs2071232 | C/T | 0.50 | China | 3016/3007 | 1.0 | 0.8–1.20 | 0.69 | Beeghly‐Fadiel et al. 57 |
| rs7125062 | C/T | 0.30 | China | 3016/3007 | 1.0 | 0.7–1.30 | 0.74 | Beeghly‐Fadiel et al. 57 |
| rs1938901 | T/C | 0.44 | China | 3016/3007 | 1.0 | 0.8–1.20 | 0.63 | Beeghly‐Fadiel et al. 57 |
| rs470747 | T/C | 0.09 | China | 3016/3007 | 0.9 | 0.3–2.10 | 0.77 | Beeghly‐Fadiel et al. 57 |
| rs2071231 | T/G | 0.21 | China | 3016/3007 | 0.7 | 0.5–1.10 | 0.98 | Beeghly‐Fadiel et al. 57 |
| rs470215 | A/G | 0.09 | China | 3016/3007 | 0.9 | 0.4–2.10 | 0.38 | Beeghly‐Fadiel et al. 57 |
| rs5854 | C/T | 0.08 | China | 3016/3007 | 0.7 | 0.2–2.10 | 0.67 | Beeghly‐Fadiel et al. 57 |
| rs7945189 | C/T | 0.07 | China | 3016/3007 | 0.7 | 0.3–1.80 | 0.64 | Beeghly‐Fadiel et al. 57 |
| rs470504 | C/T | 0.13 | China | 3016/3007 | 1.0 | 0.6–2.00 | 0.52 | Beeghly‐Fadiel et al. 57 |
| rs1939008 | A/G | 0.43 | China | 3016/3007 | 1.0 | 0.8–1.20 | 0.77 | Beeghly‐Fadiel et al. 57 |
| rs11225422 | A/G | 0.20 | China | 3016/3007 | 1.1 | 0.8–1.50 | 0.63 | Beeghly‐Fadiel et al. 57 |
| rs470226 | G/A | 0.12 | China | 3016/3007 | 0.7 | 0.4–1.30 | 0.52 | Beeghly‐Fadiel et al. 57 |
| rs7127735 | A/G | 0.21 | China | 3016/3007 | 1.1 | 0.8–1.40 | 0.41 | Beeghly‐Fadiel et al. 57 |
| rs1799750 | 1G/2G | 0.42 | United Kingdom | 126a/92b | 1.84 | 1.25–2.70 | <0.05 | Hughes et al. 58 |
| rs1799750 | 1G/2G | 0.47 | Russia | 358/746 | 1.02 | 0.85–1.22 | 0.83 | Pavlova et al. 59 |
| rs1799750 | 2G/1G | 0.46 | Russia | 239/556 | 1.00 | 0.80–1.24 | 0.99 | Pavlova et al. 60 |
| rs1799750 | 2G/1G | 0.46 | Russia | 119/190 | 1.05 | 0.75–1.45 | 0.78 | Pavlova et al. 60 |
Abbreviations: CI, confidence interval; MAF, minor allele frequency; MMP, matrix metalloproteinase; N/A, not available; OR, odds ratio.
Node positive.
Node negative.
Recent research showed that due to the differences in ethnicity, the susceptibility, incidence, and survival rate of BC varies among different populations. Besides, genetic differences also contribute to racial or ethnic variations in BC patients. To evaluate the effect of genes and their variants, a collaborative case‐control investigation in Hispanic and non‐Hispanic white females was carried out by Slattery et al. 22 They carried out a large study on 3592 BC cases and 4183 healthy controls from the United States and Mexico to examine the association of nine genetic variants including rs5854 (C/T), rs17293823 (G/A), rs996999 (C/T), rs17293761 (C/T), rs7945189 (C/T), rs7125062 (T/C), rs470358 (C/T), rs475007 (A/T), rs1144393 (T/C) in MMP1 gene with BC risk. Among them, 4 SNPs, namely, rs5854 (C/T), rs996999 (C/T), rs7125062 (T/C), and rs1144393 (T/C) were found to be significantly (p < 0.05) correlated with overall BC risk. SNP rs996999 (C/T) in the MMP1 gene showed the strongest association in females with the most Native American ancestry (odds ratio [OR] = 1.61; 95% confidence interval [CI] = 1.09–2.40; p = 0.039).
Accumulating evidence revealed that insertion of a single guanine base (1G/2G polymorphism) in the MMP1 gene promoter area generates a new binding region for the AP1 transcription factor, which attenuates the transcription level of MMP1. 61 The influence of rs1799750 1G/2G polymorphism on the MMP1 expression level, incidence, and progression of BC was investigated in a case‐control study. In that study, the genotypes and alleles distribution of the 1G/2G variant in MMP1 on 270 BC patients and 300 healthy women in the Polish population were examined. The study showed that the 2G/2G genotype and the 2G allele carriers (OR = 2.14; 95% CI = 1.24–3.69 and OR = 1.68; 95% CI = 1.19–2.39, respectively) are significantly associated (p < 0.001) with a higher possibility of axillary lymph node metastasis among BC patients. They also suggested the contribution of MMP1 to the local invasion and therefore, established MMP1 as a BC progression marker. 50 To evaluate the association of rs1799750 1G/2G variant in the progression of BC, Przybylowska et al. 62 also conducted a case‐control study on 135 subjects. They reported that 2G allele percentage was significantly greater in lymph node‐metastasis cases than in the nonmetastasis subjects (p < 0.001).
Again, a case‐control study by Padala et al. 54 showed that the promoter site genetic polymorphism of the MMP1 gene (rs1799750 1G/2G) is correlated with the development of breast carcinoma in the South Indian population. The study reported that the 2G allele of MMP1 rs1799750 had enhanced transcriptional activity. The frequency of 2G allele was also reported to be linked with twofolds enhanced risk in the patients with BC than the controls suggesting that MMP1‐1607 1G/2G gene polymorphism might have a greater association with BC.
However, a controversial relationship between MMP1 and BC risks has also been reported in different populations. The association between rs1799750 in MMP1 (1G/2G) and the risk of BC is recently investigated in a study of 598 subjects (299 BC cases and 299 healthy controls) from Poland. In this study, the results did not show any significant correlation between 1G/2G polymorphism and BC progression. 16 The 2G allele of the rs1799750 SNP in the MMP1 gene promotes the transcriptional activity of MMP1 by creating a binding region for Ets transcription factors. A study on 959 BC cases and 952 controls in the Swedish population evaluated the linkage between the MMP1 gene rs1799750 and BC. However, no statistically significant association of this polymorphism was observed with BC risk. 51 Biondi et al. 52 also observed a lack of association between MMP1 rs1799750 and BC in an Italian case‐control investigation on 43 BC cases and 164 healthy volunteers.
Notably, the MMP1 level in serum has been found to be reduced in patients with BC than the healthy subjects. Again, the 1G/2G promoter polymorphic site of MMP1 determines the levels of MMP1 influencing the susceptibility of an individual to BC. A case‐control study by Hsiao et al. 53 investigated the relationship of rs1799750 polymorphism in MMP1 to BC among the Taiwanese population. In the study, the rs1799705 polymorphic genotypes were evaluated on 1232 patients and 1232 controls but did not find any statistically significant correlation with the development of BC. The study concluded that rs1799750 may not contribute to the susceptibility of BC in the Taiwanese.
Emerging evidence showed that MMP1 is the most abundant MMP located under the control of the AP1 transcriptional factor which binds to the promoter site of mitogen‐activated protein kinase via polyomavirus enhancer activator 3 (Pea3) protein. The MMP1 expression level was found to be significantly elevated in the atypical ductal hyperplasia compared to that of the benign BC as well as in the invasive BC than that of the in situ BC. MMP1 gene rs1799750 (1G/2G) polymorphism was investigated in 1232 BC cases and 1232 healthy women from Taiwan. However, the study indicated no significant association between the 1G/2G or 1G/1G genotypes with BC susceptibility. 55
No statistically significant correlations were observed in a study in 86 BC patients and 110 controls in Italy by Ghilardi et al. 56 with the tumor, node, metastasis (TNM) stage during diagnosis of BC and between the MMP1 gene rs1799750 (1G/2G) polymorphism. The distribution of the MMP1 promoter allelic variant also demonstrated no statistically significant variations between metastatic cases and controls or between metastatic and nonmetastatic cases or between nonmetastatic cases and controls. However, they failed to establish any statistically significant link between 1G/2G polymorphism in the MMP1 gene and BC.
Another study by Balkhi 7 and colleagues examined the association between circulating levels of rs1799750 polymorphism and BC in a sample of 200 subjects. In the study, the frequencies of different genotypes for rs1799750 were observed. Among the BC cases, the frequencies were reported as 74% for the 2G/2G genotype, 2% for the 1G/2G genotype, and 24% for the 1G/1G genotype. In comparison, among the healthy volunteers, the frequencies were 38% for the 2G/2G genotype, 2% for the 1G/2G genotype, and 60% for the 1G/1G genotype. The observed differences in genotype frequencies between the two groups were found to be statistically significant (p < 0.05). Furthermore, they found that individuals with the 2G/2G genotype had a significantly increased risk of developing BC compared to those with the 1G/1G genotype (OR = 4.86; p < 0.001). They also found a significantly increased serum level of MMP1 in BC patients compared to healthy volunteers.
The study conducted by Beeghly‐Fadiel et al. 57 focused on evaluating the association between individual genetic polymorphisms across the MMP1 gene and BC risk in a two‐phase case‐control study. The study specifically included women from the Shanghai Breast Cancer Study, with a total sample size of 6023 participants. In their study, the authors investigated 23 SNPs from the MMP1 gene. However, their analysis did not find any significant correlation between these SNPs and BC risk among the participants.
In a study investigating the association between rs1799750 polymorphism of the MMP1 gene and the metastatic spread of BC, researchers evaluated 126 lymph node‐negative and 92 lymph node‐positive patients. The findings demonstrated a significant and independent association between the 2G/2G genotype and lymph node‐positive disease. For mixed ethnicities, the odds ratio was 3.9 (95% CI = 1.7–9.4), while for Caucasians, it was 2.6 (95% CI = 1.0–6.9). This suggests that individuals with the 2G/2G genotype are at an increased risk of lymph node metastasis. The study also revealed that the 2G/2G genotype was linked to reduced survival, with a hazard ratio of 3.1 (95% CI = 1.1–8.7). However, the impact on survival was dependent on lymph node status, indicating that the genotype's effect may vary depending on whether the patient has lymph node‐positive or lymph node‐negative disease. Additionally, two haplotypes of the MMP1 2G allele were significantly linked to lymph node‐positive disease and survival outcomes. 58
In a case‐control study conducted among Caucasian women in Russia, Pavlova et al. 59 investigated the association between MMP gene polymorphisms and BC risk. The study included a total of 358 affected women with BC and 746 controls. The researchers focused on 10 SNPs in five different MMP genes, including MMP1, MMP2, MMP3, MMP8, and MMP9 based on their relevance to BC from earlier studies. Although the findings of the study demonstrated a significant association between MMP gene polymorphisms and BC susceptibility in the Caucasian women of Russia, no statistically significant link was reported for MMP1 rs1799750 polymorphism. Another study published by Pavlova and others 60 in the same year (2022) explored the potential modifying effect of obesity on the association between these 10 polymorphisms and BC risk. In this study, the authors categorized the same samples (n = 1104) into two groups based on their body mass index (BMI): BMI ≥ 30 (119 BC and 190 control) and BMI < 30 (239 BC and 556 control). The findings of the study revealed an overall significant modifying effect of obesity on the association between MMP genes and BC risk in postmenopausal women. However, the authors did not find any significant link between rs1799750 and BC risk.
Some meta‐analyses 63 , 64 , 65 , 66 , 67 were also performed to evaluate the correlation between MMP1 genetic variation and the risk of BC. However, these studies failed to establish any statistically significant link between MMP1 polymorphism and BC risk, 63 , 64 , 65 , 66 except the study by Sui et al. 67 where they showed a reduced risk of BC in the heterozygous model in the overall population.
6. LITERATURE SEARCH STRATEGIES
This narrative review was conducted by following Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines for systematic review. 68 We carried out a comprehensive literature search in PubMed database (https://pubmed.ncbi.nlm.nih.gov/) as summarized in Figure 3. We have used the following search keywords: “MMP1,” “matrix metalloproteinase 1,” “MMP1 and breast cancer,” “MMP1 and carcinogenesis or malignancy,” “polymorphisms in MMP1,” “MMP1 metastasis and invasion.” We have also reviewed the bibliographic list of relevant articles to extract data from suitable ones. The literature written only in English was retrieved for our review.
Figure 3.

Flow diagram of literature search and selection for systemic review.
A total of 567 studies were collected from searching the PubMed database. After the removal of 151 duplicates, 416 articles remained for analyzing the title and abstracts. 102 articles were removed after the title and abstract screening. Excluding reviews, commentaries, not relating to MMP1 and not studying cancers except BC, a total of 122 articles remained. Due to a lack of full‐text access and inappropriate data, 95 articles were removed. One article was further excluded due to being written in other than English. Finally, 26 articles were included in the present systematic review.
7. FUTURE PERSPECTIVES
It is already established that the role of genetic polymorphism as a risk factor for cancer development is largely influenced by the ethnicity, geographical, and biological diversity of the population. 69 , 70 Moreover, the findings of genome‐wide association studies are also markedly affected by the small sample size. As a result, inconsistent results have been observed from the genetic association studies aimed to establish the correlation between MMP1 gene variants and the risk of progression of BC. In most of the studies, the published results must be validated using a larger sample size, appropriate matching between cases and controls, and unbiased investigations. Besides, a comparatively greater sample size may substantially decrease the amount of false‐positive data. 60
However, the recent advances in high‐throughput technology have permitted fast, accurate, and efficient profiling of SNPs making them appropriate choices as biomarkers for screening BC. Furthermore, understanding the effect of SNPs of a particular gene individually or in combination is very important to get the proper outcome from genetic studies. 71 Individual SNP markers alone cannot effectively provide an accurate assessment of BC risk. A combination of multiple SNPs analysis (haplotype analysis), gene‐gene interactions, understanding molecular pathways, and study with other factors including epigenetics, proteomics, posttranscriptional modifications, and others may provide the best output in future studies.
8. CONCLUSION
MMP1 is a zinc‐dependent endopeptidase that is upregulated in BC tissues and is associated with BC metastasis and invasion. The expression of MMP1 is mediated by numerous factors, including polymorphisms which act as a prominent risk factor in the progression of breast carcinogenesis. The correlation between genetic polymorphisms in MMP1 and BC risk has been analyzed in various case‐control studies in different ethnicities which is inconsistent. This is the first review concentrating on the correlation of different genetic polymorphisms in MMP1 with the risk and survival of BC as well as the structural domain of MMP1 and the role of MMP1 in BC metastasis and invasion. Our present review provides an overview of the MMP1 gene polymorphisms in BC. However, further studies are needed focusing on combined analysis of multiple SNPs, gene‐gene interactions, and analysis of epigenetics, proteomics, and posttranscriptional modifications that will provide the best outcome.
AUTHOR CONTRIBUTIONS
Tahmina Akter: data curation; formal analysis; methodology; visualization; writing—original draft. Abdul Aziz: data curation; formal analysis; methodology; visualization; writing—original draft. Mohammad Safiqul Islam: writing—review & editing. Md. Shahid Sarwar: conceptualization; supervision; writing—review & editing.
CONFLICT OF INTEREST STATEMENT
Mohammad Safiqul Islam is an Editorial Board member of Health Science Reports and a co‐author of this article. To minimize bias, he was excluded from all editorial decision‐making related to the acceptance of this article for publication.
TRANSPARENCY STATEMENT
The lead author Shahid Sarwar affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
ACKNOWLEDGMENTS
The authors are thankful to the Department of Pharmacy, Noakhali Science and Technology University, Bangladesh for the necessary support to conduct this study.
Akter T, Aziz MA, Islam MS, Sarwar MS. Association of MMP1 gene polymorphisms with breast cancer risk: a narrative review. Health Sci Rep. 2023;6:e1607. 10.1002/hsr2.1607
Tahmina Akter and Md. Abdul Aziz contributed equally.
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
REFERENCES
- 1. Jahan N, Begum M, Barek MA, et al. Evaluation of the association between FGFR2 gene polymorphisms and breast cancer risk in the Bangladeshi population. Genes. 2023;14(4):819. 10.3390/genes14040819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jafrin S, Aziz MA, Anonna SN, et al. Association of TP53 codon 72 Arg>Pro polymorphism with breast and lung cancer risk in the South Asian population: a meta‐analysis. Asian Pac J Cancer Prev. 2020;21:1511‐1519. 10.31557/APJCP.2020.21.6.1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ahmad A. Breast cancer statistics: recent trends. Adv Exp Med Biol. 2019;1152:1‐7. 10.1007/978-3-030-20301-6_1 [DOI] [PubMed] [Google Scholar]
- 4. Thorat MA, Balasubramanian R. Breast cancer prevention in high‐risk women. Best Pract Res Clin Obstet Gynaecol. 2020;65:18‐31. 10.1016/j.bpobgyn.2019.11.006 [DOI] [PubMed] [Google Scholar]
- 5. Momenimovahed Z, Salehiniya H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer (Dove Med Press). 2019;11:151‐164. 10.2147/BCTT.S176070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aziz MA, Jafrin S, Islam MS, Kabir Y. Interleukins in the Development and Progression of Breast Cancer. In: Interdisciplinary Cancer Research. Springer; 2022:1‐22. [Google Scholar]
- 7. Balkhi S, Mashayekhi F, Salehzadeh A, Saedi HS. Matrix metalloproteinase (MMP)‐1 and MMP‐3 gene variations affect MMP‐1 and ‐3 serum concentration and associates with breast cancer. Mol Biol Rep. 2020;47:9637‐9644. 10.1007/s11033-020-05962-x [DOI] [PubMed] [Google Scholar]
- 8. Lee A, Moon BI, Kim TH. BRCA1/BRCA2 pathogenic variant breast cancer: treatment and prevention strategies. Ann Lab Med. 2020;40:114‐121. 10.3343/alm.2020.40.2.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maller O, Martinson H, Schedin P. Extracellular matrix composition reveals complex and dynamic stromal‐epithelial interactions in the mammary gland. J Mammary Gland Biol Neoplasia. 2010;15:301‐318. 10.1007/s10911-010-9189-6 [DOI] [PubMed] [Google Scholar]
- 10. Quintero‐Fabián S, Arreola R, Becerril‐Villanueva E, et al. Role of matrix metalloproteinases in angiogenesis and cancer. Front Oncol. 2019;9:1370. 10.3389/fonc.2019.01370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klein T, Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids. 2011;41:271‐290. 10.1007/s00726-010-0689-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roy R, Morad G, Jedinak A, Moses MA. Metalloproteinases and their roles in human cancer. Anat Rec. 2020;303:1557‐1572. 10.1002/ar.24188 [DOI] [PubMed] [Google Scholar]
- 13. Wang X, Khalil RA. Matrix metalloproteinases, vascular remodeling, and vascular disease. Adv Pharmacol. 2018;81:241‐330. 10.1016/bs.apha.2017.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sen U. Regulation and involvement of matrix metalloproteinases in vascular diseases. Front Biosci. 2016;21:89‐118. 10.2741/4378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deryugina EI, Quigley JP. Pleiotropic roles of matrix metalloproteinases in tumor angiogenesis: contrasting, overlapping and compensatory functions. Biochimica et Biophysica Acta (BBA) ‐ Molecular Cell Research. 2010;1803:103‐120. 10.1016/j.bbamcr.2009.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Białkowska K, Marciniak W, Muszyńska M, et al. Polymorphisms in MMP‐1, MMP‐2, MMP‐7, MMP‐13 and MT2A do not contribute to breast, lung and colon cancer risk in polish population. Hereditary Cancer Clin Pract. 2020;18:16. 10.1186/s13053-020-00147-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen C, Zhang L, Jiao Y, et al. miR‐134 inhibits osteosarcoma cell invasion and metastasis through targeting MMP1 and MMP3 in vitro and in vivo. FEBS Lett. 2019;593:1089‐1101. 10.1002/1873-3468.13387 [DOI] [PubMed] [Google Scholar]
- 18. Liu J, Khalil RA. Matrix metalloproteinase inhibitors as investigational and therapeutic tools in unrestrained tissue remodeling and pathological disorders. Prog Mol Biol Transl Sci. 2017;148:355‐420. 10.1016/bs.pmbts.2017.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786‐801. 10.1038/nrm3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011;278:16‐27. 10.1111/j.1742-4658.2010.07919.x [DOI] [PubMed] [Google Scholar]
- 21. Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562‐573. 10.1016/j.cardiores.2005.12.002 [DOI] [PubMed] [Google Scholar]
- 22. Slattery ML, John E, Torres‐Mejia G, et al. Matrix metalloproteinase genes are associated with breast cancer risk and survival: the breast cancer health disparities study. PLoS One. 2013;8:e63165. 10.1371/journal.pone.0063165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tallant C, Marrero A, Gomis‐Rüth FX. Matrix metalloproteinases: fold and function of their catalytic domains. Biochim Biophys Acta, Mol Cell Res. 2010;1803:20‐28. 10.1016/j.bbamcr.2009.04.003 [DOI] [PubMed] [Google Scholar]
- 24. Chabottaux V, Noel A. Breast cancer progression: insights into multifaceted matrix metalloproteinases. Clin Exp Metastasis. 2007;24:647‐656. 10.1007/s10585-007-9113-7 [DOI] [PubMed] [Google Scholar]
- 25. Liu H, Zhang T, Li X, et al. Predictive value of MMP‐7 expression for response to chemotherapy and survival in patients with non‐small cell lung cancer. Cancer Sci. 2008;99:2185‐2192. 10.1111/j.1349-7006.2008.00922.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morgia G, Falsaperla M, Malaponte G, et al. Matrix metalloproteinases as diagnostic (MMP‐13) and prognostic (MMP‐2, MMP‐9) markers of prostate cancer. Urol Res. 2005;33:44‐50. 10.1007/s00240-004-0440-8 [DOI] [PubMed] [Google Scholar]
- 27. Waas ET, Lomme RMLM, DeGroot J, Wobbes T, Hendriks T. Tissue levels of active matrix metalloproteinase‐2 and ‐9 in colorectal cancer. Br J Cancer. 2002;86:1876‐1883. 10.1038/sj.bjc.6600366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fischer T, Senn N, Riedl R. Design and structural evolution of matrix metalloproteinase inhibitors. Chemistry. 2019;25:7960‐7980. 10.1002/chem.201805361 [DOI] [PubMed] [Google Scholar]
- 29. Cui N, Hu M, Khalil RA. Biochemical and biological attributes of matrix metalloproteinases. Prog Mol Biol Transl Sci. 2017;147:1‐73. 10.1016/bs.pmbts.2017.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Loffek S, Schilling O, Franzke CW. Biological role of matrix metalloproteinases: a critical balance. Eur Respir J. 2011;38:191‐208. 10.1183/09031936.00146510 [DOI] [PubMed] [Google Scholar]
- 31. Jabłońska‐Trypuć A, Matejczyk M, Rosochacki S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J Enzyme Inhib Med Chem. 2016;31:177‐183. 10.3109/14756366.2016.1161620 [DOI] [PubMed] [Google Scholar]
- 32. Laronha H, Caldeira J. Structure and function of human matrix metalloproteinases. Cells. 2020;9:1076. 10.3390/cells9051076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Iyer S, Visse R, Nagase H, Acharya KR. Crystal structure of an active form of human MMP‐1. J Mol Biol. 2006;362:78‐88. 10.1016/j.jmb.2006.06.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Boumiza S, Bchir S, ben Nasr H, et al. Role of MMP‐1 (‐519A/G, ‐1607 1G/2G), MMP‐3 (Lys45Glu), MMP‐7 (‐181A/G), and MMP‐12 (‐82A/G) variants and plasma MMP levels on obesity‐related phenotypes and microvascular reactivity in a Tunisian population. Dis Markers. 2017;2017:1‐16. 10.1155/2017/6198526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Trojanek J. [Matrix metalloproteinases and their tissue inhibitors]. Postepy Biochem. 2012;58:353‐362. [PubMed] [Google Scholar]
- 36. Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827‐839. 10.1161/01.RES.0000070112.80711.3D [DOI] [PubMed] [Google Scholar]
- 37. Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168:670‐691. 10.1016/j.cell.2016.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shen CJ, Kuo YL, Chen CC, Chen MJ, Cheng YM. MMP1 expression is activated by Slug and enhances multi‐drug resistance (MDR) in breast cancer. PLoS One. 2017;12:e0174487. 10.1371/journal.pone.0174487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sauter W, Rosenberger A, Beckmann L, et al. Matrix metalloproteinase 1 (MMP1) is associated with early‐onset lung cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:1127‐1135. 10.1158/1055-9965.EPI-07-2840 [DOI] [PubMed] [Google Scholar]
- 40. Brinckerhoff CE, Rutter JL, Benbow U. Interstitial collagenases as markers of tumor progression. Clin Cancer Res. 2000;6:4823‐4830. [PubMed] [Google Scholar]
- 41. Shin DH, Dier U, Melendez JA, Hempel N. Regulation of MMP‐1 expression in response to hypoxia is dependent on the intracellular redox status of metastatic bladder cancer cells. Biochim Biophys Acta. 2015;1852:2593‐2602. 10.1016/j.bbadis.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Boström P, Söderström M, Vahlberg T, et al. MMP‐1 expression has an independent prognostic value in breast cancer. BMC Cancer. 2011;11:348. 10.1186/1471-2407-11-348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang QM, Lv L, Tang Y, Zhang L, Wang LF. MMP‐1 is overexpressed in triple‐negative breast cancer tissues and the knockdown of MMP‐1 expression inhibits tumor cell malignant behaviors in vitro. Oncol Lett. 2018;17:1732‐1740. 10.3892/ol.2018.9779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tian R, Li X, Gao Y, Li Y, Yang P, Wang K. Identification and validation of the role of matrix metalloproteinase‐1 in cervical cancer. Int J Oncol. 2018;52:1198‐1208. 10.3892/ijo.2018.4267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Eiró N, González LO, Atienza S, et al. Prediction of metastatic breast cancer in non‐sentinel lymph nodes based on metalloprotease‐1 expression by the sentinel lymph node. Eur J Cancer. 2013;49:1009‐1017. 10.1016/j.ejca.2012.09.019 [DOI] [PubMed] [Google Scholar]
- 46. Eiro N, Cid S, Aguado N, et al. MMP1 and MMP11 expression in peripheral blood mononuclear cells upon their interaction with breast cancer cells and fibroblasts. Int J Mol Sci. 2020;22:371. 10.3390/ijms22010371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xuan J, Zhang Y, Zhang X, Hu F. Matrix metalloproteinase‐1 expression in breast cancer and cancer‐adjacent tissues by immunohistochemical staining. Biomed Rep. 2015;3:395‐397. 10.3892/br.2015.420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cierna Z, Mego M, Janega P, et al. Matrix metalloproteinase 1 and circulating tumor cells in early breast cancer. BMC Cancer. 2014;14:472. 10.1186/1471-2407-14-472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Coughlin SS. Epidemiology of breast cancer in women. Adv Exp Med Biol. 2019;1152:9‐29. 10.1007/978-3-030-20301-6_2 [DOI] [PubMed] [Google Scholar]
- 50. Przybylowska K, Kluczna A, Zadrozny M, et al. Polymorphisms of the promoter regions of matrix metalloproteinases genes MMP‐1 and MMP‐9 in breast cancer. Breast Cancer Res Treat. 2006;95:65‐72. 10.1007/s10549-005-9042-6 [DOI] [PubMed] [Google Scholar]
- 51. Lei H, Hemminki K, Altieri A, et al. Promoter polymorphisms in matrix metalloproteinases and their inhibitors: few associations with breast cancer susceptibility and progression. Breast Cancer Res Treat. 2007;103:61‐69. 10.1007/s10549-006-9345-2 [DOI] [PubMed] [Google Scholar]
- 52. Biondi ML, Turri O, Leviti S, et al. MMP1 and MMP3 polymorphisms in promoter regions and cancer. Clin Chem. 2000;46:2023‐2024. [PubMed] [Google Scholar]
- 53. Hsiao CL, Liu LC, Shih TC, et al. The association of matrix metalloproteinase‐1 promoter polymorphisms with breast cancer. In Vivo. 2018;32:487‐491. 10.21873/invivo.11265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Padala C, Tupurani MA, Puranam K, et al. Synergistic effect of collagenase‐1 (MMP1), stromelysin‐1 (MMP3) and gelatinase‐B (MMP9) gene polymorphisms in breast cancer. PLoS One. 2017;12:e0184448. 10.1371/journal.pone.0184448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Su CH, Lane HY, Hsiao CL, et al. Matrix metalloproteinase‐1 genetic polymorphism in breast cancer in Taiwanese. Anticancer Res. 2016;36:3341‐3345. [PubMed] [Google Scholar]
- 56. Ghilardi G, Biondi ML, Caputo M, et al. A single nucleotide polymorphism in the matrix metalloproteinase‐3 promoter enhances breast cancer susceptibility. Clin Cancer Res. 2002;8:3820‐3823. [PubMed] [Google Scholar]
- 57. Beeghly‐Fadiel A, Cai Q, Lu W, et al. No association between matrix metalloproteinase‐1 or matrix metalloproteinase‐3 polymorphisms and breast cancer susceptibility: a report from the Shanghai breast cancer study. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1324‐1327. 10.1158/1055-9965.EPI-09-0046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hughes S, Agbaje O, Bowen RL, et al. Matrix metalloproteinase single‐nucleotide polymorphisms and haplotypes predict breast cancer progression. Clin Cancer Res. 2007;13(22 Pt 1):6673‐6680. 10.1158/1078-0432.CCR-07-0884 [DOI] [PubMed] [Google Scholar]
- 59. Pavlova N, Demin S, Churnosov, M , et al. Matrix metalloproteinase gene polymorphisms are associated with breast cancer in the Caucasian Women of Russia. Int J Mol Sci. 2022;23(20):12638. 10.3390/ijms232012638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pavlova N, Demin S, Churnosov, M , et al. The modifying effect of obesity on the association of matrix metalloproteinase gene polymorphisms with breast cancer risk. Biomedicines. 2022;10(10):2617. 10.3390/biomedicines10102617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tower GB, Coon CI, Brinckerhoff CE. The 2G single nucleotide polymorphism (SNP) in the MMP‐1 promoter contributes to high levels of MMP‐1 transcription in MCF‐7/ADR breast cancer cells. Breast Cancer Res Treat. 2003;82:75‐82. 10.1023/B:BREA.0000003948.14026.7c [DOI] [PubMed] [Google Scholar]
- 62. Przybylowska K, Zielinska J, Zadrozny M, et al. An association between the matrix metalloproteinase 1 promoter gene polymorphism and lymphnode metastasis in breast cancer. J Exp Clin Cancer Res. 2004;23:121‐125. [PubMed] [Google Scholar]
- 63. McColgan P, Sharma P. Polymorphisms of matrix metalloproteinases 1, 2, 3 and 9 and susceptibility to lung, breast and colorectal cancer in over 30,000 subjects. Int J Cancer. 2009;125(6):1473‐1478. 10.1002/ijc.24441 [DOI] [PubMed] [Google Scholar]
- 64. Zhou P, Du LF, Lv GQ, et al. Current evidence on the relationship between four polymorphisms in the matrix metalloproteinases (MMP) gene and breast cancer risk: a meta‐analysis. Breast Cancer Res Treat. 2011;127(3):813‐818. 10.1007/s10549-010-1294-0 [DOI] [PubMed] [Google Scholar]
- 65. Liu D, Guo H, Li Y, Xu X, Yang K, Bai Y. Association between polymorphisms in the promoter regions of matrix metalloproteinases (MMPs) and risk of cancer metastasis: a meta‐analysis. PLoS One. 2012;7:e31251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhou Z, Ma X, Wang F, Sun L, Zhang G. A matrix metalloproteinase‐1 polymorphism,MMP1–1607(1G>2G), is associated with increased cancer risk: a meta‐analysis including 21,327 patients. Dis Markers. 2018;2018:7565834. 10.1155/2018/7565834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sui J, Huang J, Zhang Y. The MMP‐1 gene rs1799750 polymorphism is associated with breast cancer risk. Genet Test Mol Biomarkers. 2021;25(7):496‐503. 10.1089/gtmb.2021.0016 [DOI] [PubMed] [Google Scholar]
- 68. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Datta A, Tuz Zahora F, Abdul Aziz M, et al. Association study of IL10 gene polymorphisms (rs1800872 and rs1800896) with cervical cancer in the Bangladeshi women. Int Immunopharmacol. 2020;89:107091. 10.1016/j.intimp.2020.107091 [DOI] [PubMed] [Google Scholar]
- 70. Huang T, Shu Y, Cai YD. Genetic differences among ethnic groups. BMC Genomics. 2015;16:1093. 10.1186/s12864-015-2328-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Suhaimi SA, Chan SC, Rosli R. Matrix metallopeptidase 3 polymorphisms: emerging genetic markers in human breast cancer metastasis. J Breast Cancer. 2020;23:1‐9. 10.4048/jbc.2020.23.e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.


