Figure 1.
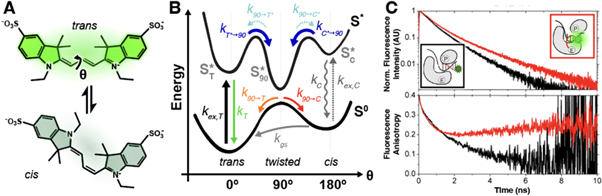
PIFE concepts. (A) Molecular structure of the cyanine dye sCy3 as trans (top) and cis isomer (bottom). Isomerisation along the polymethine chain modulates the fluorescence of sCy3. (B) Energy diagram of sCy3 as a function of the rotation coordinate θ in the trans (0o) and cis (180o) state. Upon excitation into the excited trans state (), deactivation occurs upon internal conversion and fluorescence (summarized by the decay rate k T) or by isomerisation (k T*→90) into the twisted state (90o). The excited cis state () decays via internal conversion to the cis ground-state with a decay rate (k C) or by isomerisation (k C*→90). From the excited-state minimum in the twisted state, sCy3 forms the trans and cis ground-state with rates k 90→T or k 90→C, respectively. In the ground-state, the reconversion from cis to trans isomer is again thermally driven with a rate kgs. Adapted from Lerner, Ploetz et al. [18] under the terms of the Creative Commons CC-BY License 4.0. (C) Using smPIFE at the single-molecule level allows, for example, monitoring the position of a Cy3-labelled dsDNA construct outside (black) and inside (red) a Klenow fragment via time-resolved fluorescence (top) and anisotropy (bottom). The transition of the primer to the exonuclease site pulls the Cy3-labelled fragment from a solvent-exposed to protein-surrounded position, leading to a change in environment detected by PIFE. License: C) Adapted with permission from {Stennett E M S, Ciuba M A, Lin S and Levitus M 2015 Demystifying PIFE: The Photophysics Behind the Protein-Induced Fluorescence Enhancement Phenomenon in Cy3 The Journal of Physical Chemistry Letters 6 1819–23} [19]. Copyright {2015} American Chemical Society.
