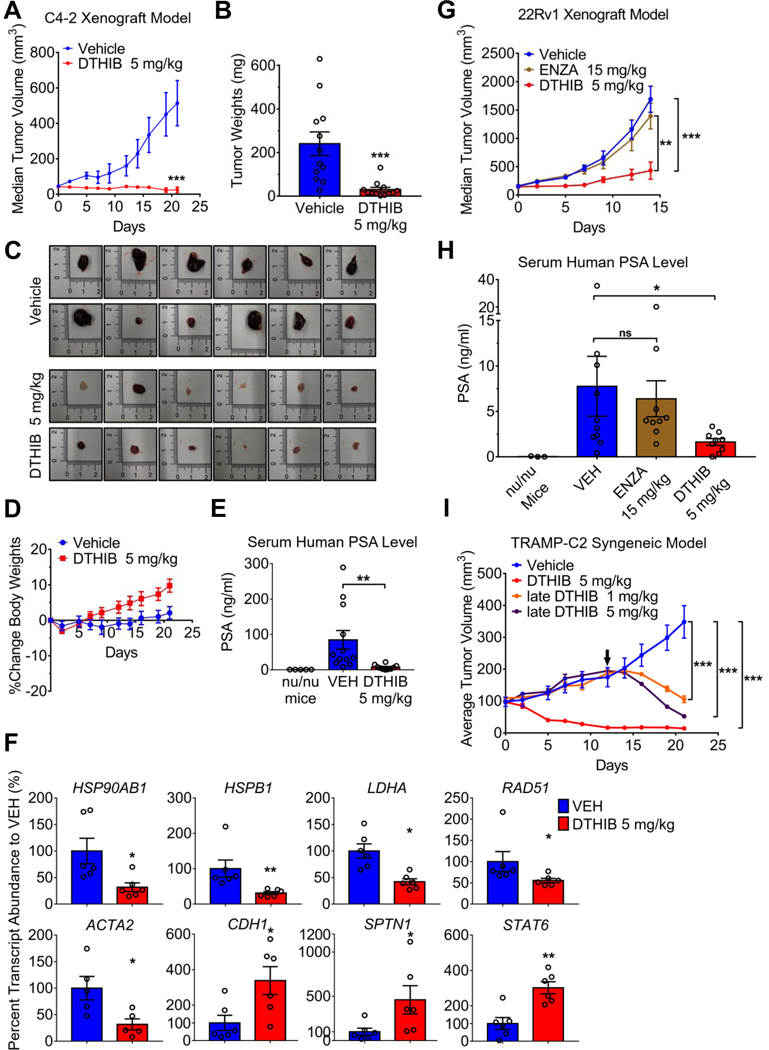
Fig. 6.
Pharmacological inhibition of HSF1 attenuates prostate cancer progression in animal models. (A) Median tumor volume plot over 21 days of C4–2 xenograft study in which vehicle (blue) or DTHIB (red) at 5 mg/kg were administered daily by IP to independent mouse cohorts (n = 12). (B) Tumors from the C4–2 xenograft study (A) were excised from mice and weighed at the experimental endpoint (n = 12/cohort). (C) Images of tumors from the C4–2 xenograft study (A) harvested at the experimental endpoint. (D) Percent change in average body weight of mice from the C4–2 xenograft study (A) (n = 12/cohort). (E) Mouse serum samples from the C4–2 xenograft study (A) obtained at the experimental endpoint were assayed for human PSA levels by ELISA (n = 12/cohort), with serum from nu/nu mice (no tumor implantation and no drug treatment) as negative control (n = 5). (F) Pharmacodynamic analysis of transcript abundance corresponding to direct HSF1 target genes in C4–2 tumors (n = 6/cohort), by qRT-PCR. CDH2, SPTN1 and STAT6 are genes repressed by HSF1, all others are genes activated by HSF1. (G) Median tumor volume plot from a 22Rv1 xenograft study in which vehicle, enzalutamide (ENZA) at 15 mg/kg or DTHIB at 5 mg/kg were administered daily by IP to three animal cohorts (n = 10/cohort). (H) At the experimental endpoint, mouse serum samples (n = 10/cohort) from 22Rv1 xenograft study (G) were collected and assayed for human PSA levels by ELISA, using nu/nu mouse serum as blank (n = 3). (I) In the TRAMP-C2 syngeneic mouse prostate cancer model tumors from two cohorts of 8 mice/cohort were allowed to grow to an average tumor volume of 100 mm3 and mice then treated with vehicle or DTHIB at 5 mg/kg daily by IP. Tumors from two other cohorts of 4 mice/cohort (late cohorts) were allowed to grow to an average tumor volume of ~200 mm3, and treated with 5 mg/kg DTHIB or 1 mg/kg DTHIB daily by IP. Arrow indicated the treatment start date of the “late” cohorts.