Abstract
Background:
Patients with pre-existing rheumatoid arthritis initiating immune checkpoint inhibitors for cancer might be at risk of increased mortality, rheumatoid arthritis flares, and other immune-related adverse events (AEs). We aimed to determine whether pre-existing rheumatoid arthritis was associated with higher mortality and immune-related AE risk in patients treated with immune checkpoint inhibitors.
Methods:
This retrospective, comparative cohort study was conducted at the Mass General Brigham Integrated Health Care System and the Dana-Farber Cancer Institute in Boston (MA, USA). We searched data repositories to identify all individuals who initiated immune checkpoint inhibitors from April 1, 2011, to April 21, 2021. Patients with pre-existing rheumatoid arthritis had to meet the 2010 American College of Rheumatology–European Alliance of Associations for Rheumatology (ACR–EULAR) criteria. For each pre-existing rheumatoid arthritis case, we matched up to three non-rheumatoid arthritis comparators at the index date of immune checkpoint inhibitor initiation by sex (recorded as male or female), calendar year, immune checkpoint inhibitor target, and cancer type and stage. The coprimary outcomes were time from index date to death and time to the first immune-related AE, measured using an adjusted Cox proportional hazards model. Deaths were identified by medical record and obituary review. Rheumatoid arthritis flares and immune-related AE presence, type, and severity were determined by medical record review.
Findings:
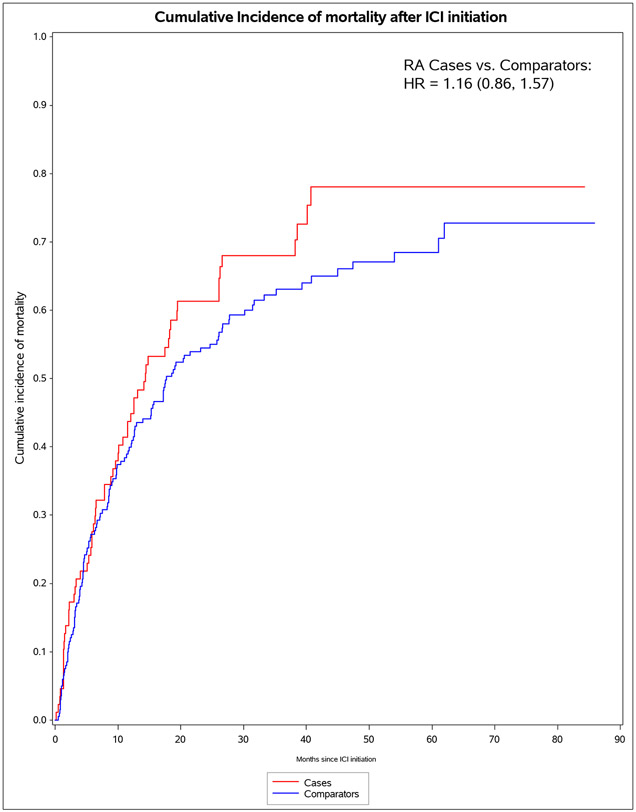
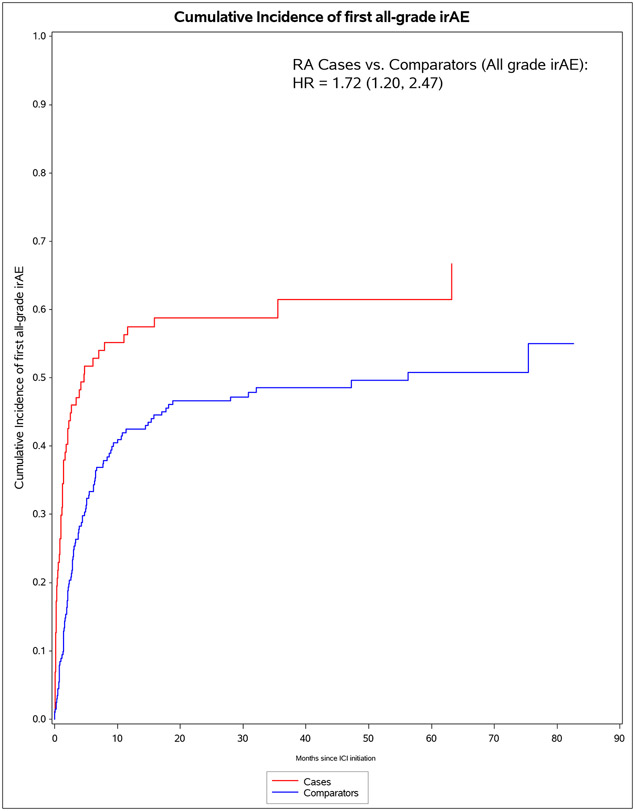
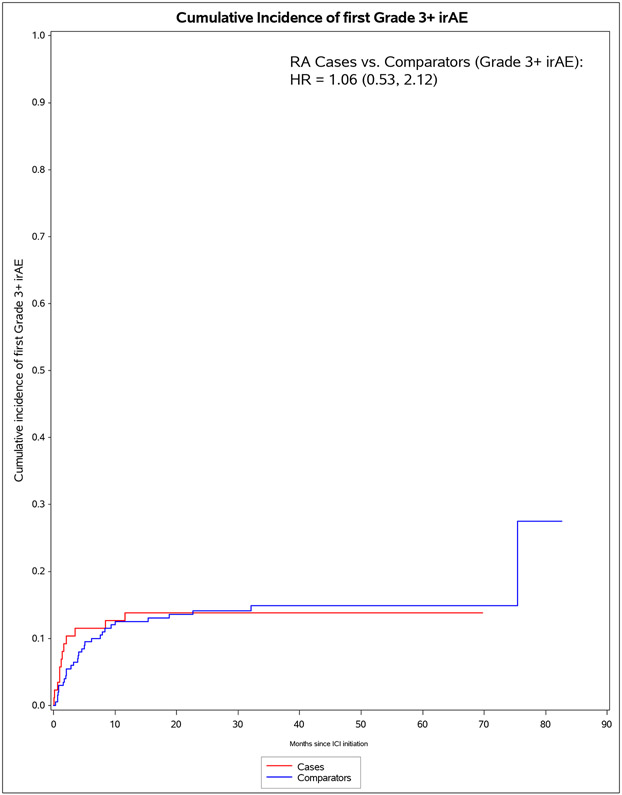
We identified 11 901 patients who initiated immune checkpoint inhibitors for cancer treatment between April 1, 2011, and April 21, 2021; of those, 101 met the 2010 ACR–EULAR criteria for rheumatoid arthritis. We successfully matched 87 patients with pre-existing rheumatoid arthritis to 203 non-rheumatoid arthritis comparators. The median age was 71·2 years (IQR 63·2–77·1). 178 (61%) of 290 participants were female, 112 (39%) were male and 268 (92%) participants were White. PD-1 was the most common immune checkpoint inhibitor target (80 [92%] of 87 patients with rheumatoid arthritis vs 188 [93%] of 203 comparators). Lung cancer was the most common cancer type (43 [49%] vs 114 [56%]), followed by melanoma (21 [24%] vs 50 [25%]). 60 (69%) patients with rheumatoid arthritis versus 127 (63%) comparators died (adjusted hazard ratio [HR] of 1·16 [95% CI 0·86–1·57]; p=0·34). 53 (61%) patients with rheumatoid arthritis versus 99 (49%) comparators had any all-grade immune-related AE (adjusted HR 1·72 [95% CI 1·20–2·47]; p=0·0032). There were two (1%) grade 5 immune-related AEs (deaths) due to myocarditis, both in the comparator group. Rheumatoid arthritis flares occurred in 42 (48%) patients with rheumatoid arthritis, and inflammatory arthritis occurred in 14 (7%) comparators (p<0·0001). Those with rheumatoid arthritis were less likely to have rash or dermatitis (five [6%] vs 28 [14%]; p=0·048), endocrinopathy (two [2%] vs 22 [11%]; p=0·0078), colitis or enteritis (six [7%] vs 28 [14%] comparators; p=0·094), and hepatitis (three [3%] vs 19 [9%]; p=0·043).
Interpretation:
Patients with pre-existing rheumatoid arthritis initiating immune checkpoint inhibitors had similar risk of mortality and severe immune-related AEs as matched comparators. Although patients with pre-existing rheumatoid arthritis were more likely to have immune-related AEs, this finding was mostly due to mild rheumatoid arthritis flares. These results suggest that this patient population can safely receive immune checkpoint inhibitors for cancer treatment.
Funding:
None
INTRODUCTION
Immune checkpoint inhibitors (ICI) stimulate the immune system to attack cancer cells, leading to improved survival1-4. However, ICI can also cause immune-related adverse events (irAE) that affect nearly every organ system5. While most irAE are mild, severe forms can result in significant morbidity and mortality6-8 and may require ICI disruption and necessitate treatment with systemic immunosuppression.
Patients with pre-existing autoimmune diseases, such as rheumatoid arthritis (RA), at ICI initiation may be at increased risk for irAE or flares of their underlying disease9-11. Patients with RA were excluded from ICI clinical trials due to concerns regarding their potential for severe irAE and baseline need for immunosuppression that may blunt ICI efficacy12,13. Most previous studies focused on all pre-existing autoimmune conditions9,14,15, and many lacked comparator groups. There have been few studies that included only pre-existing RA patients initiating ICI16. Focusing only on pre-existing RA for ICI outcomes may limit the heterogeneity across autoimmune diseases for ICI outcomes. For example, pre-existing autoimmune thyroid disease is common but is not treated with systemic immunosuppression, whereas immunosuppressive therapies are typically required for RA. It is possible that pre-existing RA may affect mortality risk in the setting of ICI treatment due to the need for systemic immunosuppression, leading to disruption of ICI therapy, or propensity for RA flares or severe irAE.
Therefore, we studied patients at Mass General Brigham (MGB)/Dana-Farber Cancer Institute (DFCI) with pre-existing RA who received ICI for cancer treatment, also including matched comparators. We investigated whether pre-existing RA was associated with higher mortality and irAE risk than comparators.
METHODS
Study design and population
We performed a retrospective comparative cohort study investigating mortality and irAE outcomes after ICI initiation for cancer treatment at MGB, a tertiary health care system, and the Dana-Farber Cancer Institute (DFCI). Both MGB and DFCI are located in the greater Boston, Massachusetts area and share the same electronic health record (EHR). We performed electronic query of the data repositories of each institution, the Research Patient Data Registry at MGB and the Oncology Data Retrieval System at DFCI, to identify all individuals who initiated an ICI from 1/Apr/2011 to 21/Apr/2021. The index date was the date of first prescription for ICI, medications targeting PD-1 (programmed cell death protein-1: pembrolizumab/nivolumab/cemiplimab), PD-L1 (programmed death-ligand 1: atezolizumab/avelumab/durvalumab), or CTLA-4 (cytotoxic T-lymphocyte-associated protein-4: ipilimumab/tremelimumab). We included all patients initiating an ICI for cancer treatment, irrespective of concomitant chemotherapy or any other cancer treatment. Clinical trial patients were included if ICI was not the intervention since we could not have determined whether patients were on active drug or placebo. This study was approved by the institutional review boards of MGB and DFCI. Patient informed consent was not required for this retrospective study.
Identification of pre-existing RA cases
The exposure variable was pre-existing RA case vs matched comparator status. We identified pre-existing RA cases using a 2-stage process. First, we identified all patients who had at least one International Classification of Diseases 9th or 10th edition for inflammatory arthritis (IA)5 (Appendix pp.4-6) prior to or on the index date of ICI initiation as a sensitive screen. We did not consider codes after the index date since these may have been used for patients experiencing IA as an irAE, rather than for pre-existing RA. Second, we performed detailed medical record review by at least two trained study physicians to confirm pre-existing RA before ICI initiation that met 2010 ACR/EULAR criteria17.
Baseline RA characteristics
We collected data on RA characteristics at time of ICI initiation for descriptive purposes, using medical record review and electronic query. Characteristics included RA duration, serostatus (seropositivity defined as elevated anti-cyclic citrullinated peptide [anti-CCP] and/or rheumatoid factor [RF]), most recent RA disease activity from the most recent clinical visit with their rheumatologist within one year before index date (categorized as remission/low/moderate/high from validated measures18 or from physician global assessment), and medication use (glucocorticoids and disease-modifying antirheumatic drugs [DMARDs]). We collected data on bone erosions, rheumatoid nodules, deformities, interstitial lung disease (ILD)19, secondary Sjögren syndrome, rheumatoid vasculitis, and Felty’s syndrome.
Matched non-RA comparators
For each pre-existing RA case, we identified up to 3 matched non-RA comparators who had initiated an ICI. Matching factors were as of the index date of ICI initiation and included: age (+/− 5 years), sex, calendar year (+/− 1 year), ICI target (PD-1, PD-L1, CTLA-4, or combination), and cancer type/stage. We chose these matching factors since they may have confounded associations with pre-existing RA and outcomes. The pool of eligible non-RA comparators had no ICD-9/10 codes for IA or any other systemic rheumatic disease (as previously detailed5) prior to the index date. Verification of cancer type/stage and mortality/irAE outcomes required medical record review to verify matches and outcomes. As detailed5, we identified the cancer type through histologic diagnosis on a majority of patients that had these data from their primary tumor in the DFCI database. If histologic type was unavailable, we used ICD-9/10 codes from the EHR to classify cancer type5. Finally, we verified that all pre-existing RA cases and comparators in analyses were exact matches for histologic cancer type/stage using medical record review. There were some pre-existing RA cases with uncommon cancers for whom we could not identify suitable comparators.
All-cause mortality and end of follow-up
The first co-primary outcome was time from index date to death in an adjusted Cox proportional hazards model. We used electronic query to identify vital status, including presence and date of death. Since out-of-hospital deaths may not be accurately documented in our EHR, we also queried obituaries. We documented the last clinical encounter that verified vital status as the end of follow-up for each patient.
RA flare and irAE outcomes
The second co-primary outcome was time from index date to first irAE in an adjusted Cox proportional hazards model. Secondary outcomes were: number of irAE, severe irAE, and specific types of irAE. Another analysis did not consider RA flares for cases or IA for comparators as part of the irAE definition to examine non-RA/IA irAE types and severity. We performed retrospective medical record review to identify presence, type, severity, and date of RA flares and irAE occurring after the index date of ICI initiation. Not all patients were evaluated by rheumatologists for RA flares since many were diagnosed and treated by oncologists. We considered the following categories of irAE5: RA flare (for cases) or IA (for comparators), rash/dermatitis, colitis/enteritis, endocrinopathy (hypo/hyperthyroidism, adrenal, pituitary, type 1 diabetes), hepatitis, pneumonitis, hematologic (cytopenias, autoimmune hemolytic anemia, immune thrombocytopenic purpura, hemolytic uremic syndrome, aplastic anemia), other rheumatologic (polymyalgia rheumatica [PMR]-like syndrome, lupus, vasculitis, sicca syndrome), myocarditis/pericarditis, myositis, other neurologic (autonomic, neuropathy, meningitis, cerebritis, transverse myelitis, Guillain-Barré syndrome), myasthenia gravis, nephritis, ocular (uveitis/iritis/episcleritis/blepharitis), pancreatitis, vitiligo, or other. Highest severity of each irAE type was graded from 1 (least severe) to 5 (death) for each irAE using standardized criteria specific to each irAE type5. We considered grade 3 or higher as a severe irAE20.
Covariates
Data on age, sex, race, ethnicity, body mass index, and the Charlson Comorbidity Index21 (CCI) were obtained from electronic query. For the CCI, we included ICD-9/10 codes occurring within 1 year before index date. We obtained all other covariate data using medical record review. We collected data on smoking status (never/past/current) and continuous pack-years as of the index date. We grouped the cancer type based on the primary organ involved (lung, melanoma, genitourinary tract, gastrointestinal tract, head/neck, hematologic, brain, and other). We collected cancer duration and previous chemotherapy, hormonal therapy, radiation, stem cell transplant, or chimeric antigen receptor T cell (CAR T) therapy.
Statistical analysis
We displayed baseline characteristics of pre-existing RA cases and non-RA comparators using descriptive statistics. We compared RA cases and comparators using univariate tests. Since we expected RA flares to be common among the pre-existing RA cases and the pathogenesis may differ, we pre-specified analyses excluding RA flare and IA events to investigate risk of irAE other than IA. We compared the presence of all-grade and grade 3+ irAE for each specific irAE. For descriptive purposes, we reported the types of immunosuppression received as well as number and duration of ICI after index date in RA cases and comparators. We did not adjust for these post-baseline variables since they may have mediated associations or in response to irAE events.
We performed time-to-event analyses, comparing pre-existing RA cases and comparators for the outcomes of all-cause mortality, all-grade irAE, and grade 3+ irAE. We used Cox regression to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for mortality. Person-time accrued as of the date of ICI initiation and the censoring events were dates of death or last follow-up. We calculated IRs (IR) and 95%CIs per 1000 person-months. The base model was unadjusted, but cases and comparators were controlled for matching factors. The multivariable models were additionally adjusted for continuous smoking pack-years, continuous cancer duration, previous cancer treatment, and continuous CCI. For the irAE analyses, the censoring events were date of initial irAE being analyzed, date of death, or date of last follow-up, whichever came first. We also performed competing risk analyses when investigating irAE to account for the possible differential mortality. We used the Fine and Gray method22 to obtain subdistribution (sd) HRs and 95%CIs for irAE risk, with unadjusted and multivariable analyses as in the Cox models.
We performed some secondary and subgroup analyses. We reported the associations of covariates with mortality and stratified by the following subgroups: males, females, lung cancer, melanoma, seropositive RA, seronegative RA, RA on any DMARD at index date, RA not on any DMARD at index date, and RA on or not on glucocorticoids at index date. We also performed stratified analyses by male or female sex for all irAE outcomes. We stratified the analysis by before 2016 vs. 2016 or later to investigate possible differences by calendar time. We also performed subgroup analyses based on moderate/high vs. remission/low RA disease activity at baseline. There were no missing data on covariates included in multivariable models. For subgroup analyses based on RA characteristics such as serostatus and disease activity, those with missing data were not analyzed.
The proportional hazards assumption was met in all analyses by confirming no statistical interaction between case/comparator status and follow-up time for each outcome. Two-sided p<0.05 was considered statistically significant. Analyses were performed using SAS v.9.4 (Cary, NC).
Role of the funding source
There was no funding source.
RESULTS
We identified 11,901 patients from who initiated an ICI for cancer treatment, and 101 patients met 2010 ACR/EULAR criteria for RA before ICI initiation (Appendix p.3). We successfully matched 87 of these pre-existing RA cases to 203 non-RA comparators by age, sex, calendar year, cancer type/stage, and ICI target.
Mean age was 69.9 vs 69.4 years and 52/87 (60%) vs 126/203 (62%) were female in pre-existing RA cases and comparators, respectively (Table 1). Of pre-existing RA cases, 81/87 (93%) were White vs 187/203 (92%) of comparators. The median smoking pack-years was 20 for cases and comparators. PD-1 was the most common ICI target (80/87 [92%] vs 188/203 [93%]). Lung cancer was the most common cancer type (43/87 [49%] vs 114/203 [56%]), followed by melanoma (21/87 [24%] vs 50/203 [25%]). Pre-existing RA cases were more likely to have received any previous cancer treatment (53/87 [61%] vs 94/203 [46%], p=0.023).
Table 1.
Baseline characteristics of pre-existing RA cases (n=87) and matched non-RA comparators (n=203) at index date of immune checkpoint inhibitor initiation.
| Pre-existing RA cases (n=87) |
Matched non-RA comparators (n=203) |
p-value | |
|---|---|---|---|
| Demographics | |||
| Mean age, years (SD) | 69.9 (10.6) | 69.4 (10.4) | 0.76* |
| Sex, n (%) | |||
| Female | 52 (60%) | 126 (62%) | 0.71* |
| Male | 35 (40%) | 77 (38%) | |
| Race, n (%)** | |||
| White | 81 (93%) | 187 (92%) | 0.96 |
| Black | 3 (3%) | 7 (3%) | |
| Asian | 1 (1%) | 2 (1%) | |
| Median calendar year (IQR) | 2018 (2017, 2019) | 2018 (2017, 2019) | 0.42* |
| Lifestyle | |||
| Smoking status, n (%) | |||
| Never | 24 (29%) | 51 (25%) | 0.56 |
| Past | 57 (66%) | 130 (64%) | |
| Current | 6 (7%) | 22 (11%) | |
| Median pack-years (IQR) | 20 (0, 40) | 20 (0, 40) | 0.90 |
| Median body mass index, kg/m2 (IQR) | 25.8 (22.0, 29.4) | 26.0 (23.3, 31.0) | 0.065 |
| Comorbidities | |||
| Median CCI (IQR) | 8 (6, 9) | 8 (3, 10) | 0.44 |
| Cancer characteristics | |||
| Target of ICI, n (%) | 0.94* | ||
| PD-1 | 80 (92%) | 188 (93%) | |
| PD-L1 | 4 (5%) | 9 (4%) | |
| CTLA-4 | 1 (1%) | 1 (1%) | |
| Combination | 2 (2%) | 5 (3%) | |
| Type of cancer, n (%) | |||
| Lung | 43 (49%) | 114 (56%) | 0.29* |
| Non-small cell | 41 (47.1%) | 112 (55%) | 0.21* |
| Small cell | 2 (2%) | 2 (1%) | 0.27* |
| Melanoma | 21 (24%) | 50 (25%) | 0.93* |
| Genitourinary tract | 6 (7%) | 12 (6%) | 0.75* |
| Gastrointestinal tract | 3 (4%) | 6 (3%) | 0.27* |
| Head and neck | 4 (5%) | 7 (4%) | 0.22* |
| Hematologic | 3 (4%) | 3 (2%) | 0.19* |
| Brain | 2 (2%) | 5 (3%) | 0.32* |
| Other*** | 5 (6%) | 6 (%) | 0.25* |
| Median cancer duration, years (IQR) | 0.9 (0.1, 2.4) | 0.6 (0.1, 1.9) | 0.66 |
| Previous chemotherapy, n (%) | 53 (61%) | 91 (45%) | 0.012 |
| Previous hormonal therapy, n (%) | 1 (1%) | 4 (2%) | 0.36 |
| Previous radiation, n (%) | 48 (55%) | 71 (35%) | 0.0014 |
| Previous stem cell transplant, n (%) | 2 (2%) | 2 (1%) | 0.267 |
| Previous CAR-T therapy, n (%) | 0 (0%) | 1 (1%) | 0.70 |
| Any previous chemotherapy, hormonal therapy, radiation, stem cell transplant, or CAR-T, n (%) | 53 (61%) | 94 (46%) | 0.023 |
| RA characteristics | |||
| Median RA duration, years (IQR) | 9.4 (4.6, 16.5) | ||
| Seropositive, n (%) | 49/71 (69%) | ||
| Anti-CCP+, n (%) | 35/57 (61%) | ||
| RF+ | 37/58 (64%) | ||
| Most recent disease activity, n (%) | |||
| Remission | 29/68 (43%) | ||
| Low | 25/68 (37%) | ||
| Moderate | 11/68 (16%) | ||
| High | 3/68 (4%) | ||
| Glucocorticoid (n, %) | 57 (66%) | ||
| Median prednisone dose, mg/day (IQR) | 10 (5, 26) | ||
| Any DMARD (n, %) | 40 (46%) | ||
| Any csDMARD (n, %) | 31 (36%) | ||
| Methotrexate (n, %) | 19 (22%) | ||
| Hydroxychloroquine (n, %) | 11 (13%) | ||
| Any bDMARD or tsDMARD (n, %) | 22 (25%) | ||
| TNFi (n, %) | 10 (12%) | ||
| Bone erosions or deformities, n (%) | 27 (31%) | ||
| Interstitial lung disease, n (%) | 13 (15%) | ||
| Rheumatoid vasculitis, n (%) | 1 (1%) | ||
| Sjogren’s syndrome, n (%) | 0 (0.0%) | ||
| Felty’s syndrome, n (%) | 0 (0.0%) |
Some percentages may not total 100% due to rounding. Missing data: race, 2 cases and 7 comparators; BMI, 9 cases and 20 comparators. RA characteristics- serostatus 16; anti-CCP 30; RF status 29; disease activity 19. All other variables had complete data.
Age, sex, calendar year, ICI target, and cancer type/stage were matching factors.
No patients reported other races or Hispanic/Latinx ethnicity.
Other cancers included neuroendocrine (2 cases and 2 comparators), Merkel cell carcinoma (2 cases and 3 comparators), and mesothelioma (1 case and 1 comparator).
CAR-T, chimeric antigen receptor T cells; CCI, Charlson Comorbidity Index; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; ICI, immune checkpoint inhibitor; IQR, interquartile range; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; RA, rheumatoid arthritis; SD, standard deviation.
The median RA duration was 9.4 years; 49/71 (69%) with serologic data were seropositive (Table 1). Most were in remission (29/68, [43%]) or in low disease activity (25/68 [37%]) at ICI initiation; 57/87 (66%) were taking glucocorticoids, and 40/87 (46%) were taking DMARDs. Characteristics of pre-existing RA cases included in the analysis vs. those excluded due to no match are included in Appendix pp.3-4.
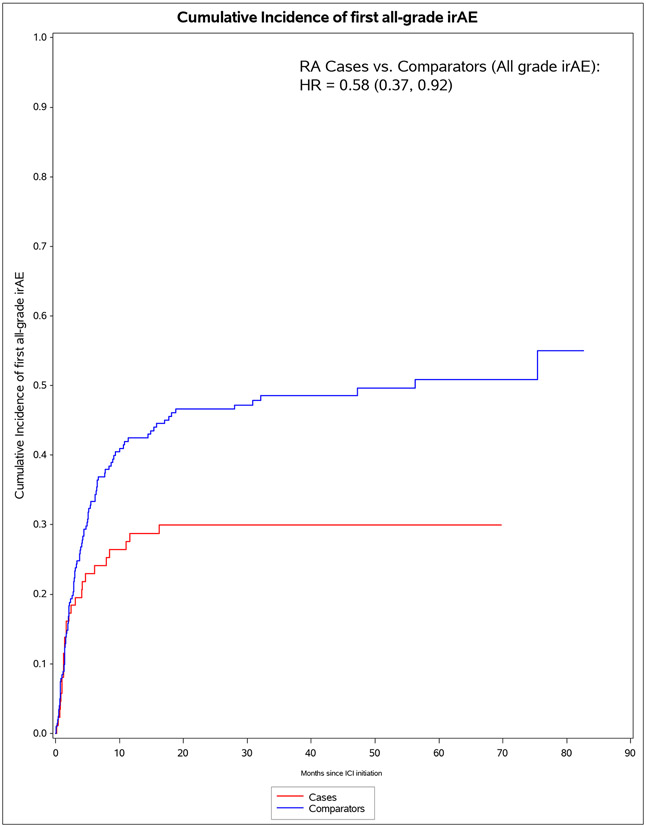
A total of 60/87 (69%) of pre-existing RA cases died vs 127/203 (63%) of comparators died (p=0.30, Table 2). For irAE, 53/87 (61%) of pre-existing RA cases vs 99/203 (49%) experienced any all-grade irAE (p=0.058). When considering only grade 3+ irAE, 12/87 (14%) of pre-existing RA cases had severe irAE compared to 30/203 (15%) of comparators (p=0.83). There were two grade 5 irAE (deaths), each due to myocarditis among comparators. In the analysis not considering RA flares/IA as irAE events, pre-existing RA cases were less likely to experience any all-grade irAE, 26/87 (30%) vs 99/203 (49%, p=0.0029). Pre-existing RA cases were also less likely to experience multiple irAE (9/87 [10%] vs 42/203 [21%], p=0.034).
Table 2.
Mortality and irAE presence and type after immune checkpoint inhibitor initiation for pre-existing RA cases (n=87) and comparators (n=203).
| Pre-existing RA cases (n=87) |
Matched non-RA comparators (n=203) |
p-value | |
|---|---|---|---|
| All-cause mortality | 60 (69%) | 127 (63%) | 0.30 |
| All RA flares and irAE | |||
| Any all-grade irAE | 53 (61%) | 99 (49%) | 0.058 |
| Any grade 3+ irAE | 12 (14%) | 30 (15%) | 0.83 |
| Any grade 4+ irAE | 4 (5%) | 6 (3%) | 0.20 |
| Any grade 5 irAE | 0 (0%) | 2 (1%) | 0.49 |
| Median and IQR number of all-grade irAE | 1 (0, 1) minimum 0 maximum 4 |
0 (0, 1) minimum 0 maximum 5 |
0.45 |
| Median and IQR number of grade 3+ irAE | 0 (0, 0) minimum 0 maximum 3 |
0 (0, 0) minimum 0 maximum 4 |
0.73 |
| Exactly one all-grade irAE | 36 (41%) | 57 (28%) | 0.026 |
| Two or more all-grade irAE | 17 (20%) | 42 (21%) | 0.82 |
| Excluding RA flares for cases and inflammatory arthritis events for comparators | |||
| Any all-grade irAE | 26 (30%) | 99 (49%) | 0.0029 |
| Any grade 3+ irAE | 11 (13%) | 30 (15%) | 0.63 |
| Median and IQR number of all-grade irAE* | 0 (0, 1) minimum 0 maximum 3 |
0 (0, 1) minimum 0 maximum 5 |
0.0018 |
| Median and IQR number of grade 3+ irAE | 0 (0, 0) minimum 0 maximum 2 |
0 (0, 0) minimum 0 maximum 4 |
0.4586 |
| Exactly one all-grade irAE | 17 (20%) | 57 (28%) | 0.13 |
| Two or more all-grade irAE | 9 (10%) | 42 (21%) | 0.034 |
| Specific irAE types | |||
| RA flare (for cases) or inflammatory arthritis (for comparators) | |||
| All-grade | 42 (48%) | 14 (7%) | <0.0001 |
| Grade 3+ | 2 (2%) | 0 (0%) | 0.089 |
| Rash/dermatitis | |||
| All-grade | 5 (6%) | 28 (14%) | 0.048 |
| Grade 3+ | 1 (1%) | 1 (1%) | 0.42 |
| Colitis/enteritis | |||
| All-grade | 6 (7%) | 28 (14%) | 0.094 |
| Grade 3+ | 1 (1%) | 10 (5%) | 0.090 |
| Endocrinopathy (hypo/hyperthyroidism, adrenal, pituitary, type 1 diabetes) | |||
| All-grade | 2 (2%) | 22 (11%) | 0.0078 |
| Grade 3+ | 0 (0%) | 3 (2%) | 0.34 |
| Hepatitis | |||
| All-grade | 3 (4%) | 19 (9%) | 0.043 |
| Grade 3+ | 1 (1%) | 6 (3%) | 0.25 |
| Pneumonitis | |||
| All-grade | 9 (10%) | 19 (9%) | 0.79 |
| Grade 3+ | 4 (5%) | 6 (3%) | 0.20 |
| Hematologic (cytopenias, autoimmune hemolytic anemia, immune thrombocytopenic purpura, hemolytic uremic syndrome, aplastic anemia) | |||
| All-grade | 2 (2%) | 4 (2%) | 0.33 |
| Grade 3+ | 1 (1%) | 1 (1%) | 0.42 |
| Other rheumatologic (PMR-like syndrome, lupus, vasculitis, sicca syndrome) | |||
| All-grade | 1 (1%) | 6 (3%) | 0.24 |
| Grade 3+ | 0 (0%) | 1 (1%) | 0.70 |
| Myocarditis/pericarditis | |||
| All-grade | 1 (1%) | 4 (2%) | 0.36 |
| Grade 3+ | 1 (1%) | 4 (2%) | 0.36 |
| Myositis | |||
| All-grade | 0 (0%) | 2 (1%) | 0.49 |
| Grade 3+ | 0 (0%) | 1 (1%) | 0.70 |
| Other neurologic (autonomic, neuropathy, meningitis, cerebritis, transverse myelitis, Gullain-Barré syndrome) | |||
| All-grade | 2 (2%) | 5 (3%) | 0.32 |
| Grade 3+ | 1 (1%) | 2 (1%) | 0.44 |
| Myasthenia gravis | |||
| All-grade | 0 (0%) | 1 (1%) | 0.70 |
| Grade 3+ | 0 (0%) | 1 (1%) | 0.70 |
| Nephritis | |||
| All-grade | 1 (1%) | 3 (2%) | 0.41 |
| Grade 3+ | 1 (1%) | 1 (1%) | 0.42 |
| Ocular (uveitis/iritis/episcleritis/blepharitis) | |||
| All-grade | 1 (1.2%) | 1 (0.5%) | 0.42 |
| Grade 3+ | 0 (0.0%) | 0 (0.0%) | - |
| Pancreatitis | |||
| All-grade | 1 (1.2%) | 2 (1.0%) | 0.44 |
| Grade 3+ | 1 (1.2%) | 0 (0.0%) | 0.30 |
| Vitiligo | |||
| All-grade | 2 (2%) | 3 (2%) | 0.31 |
| Grade 3+ | 0 (0%) | 0 (0%) | - |
| Other | |||
| All-grade | 0 (0%) | 1 (1%) | 0.70 |
| Grade 3+ | 0 (0%) | 0 (0%) | N/A |
See Appendix p.12 for a histogram comparing number of irAE for pre-existing RA cases and non-RA comparators after excluding RA flares for cases and IA events for comparators.
IA, inflammatory arthritis; IQR, interquartile range; irAE, immune-related adverse event; PMR, polymyalgia rheumatica; RA, rheumatoid arthritis.
When evaluating specific types of irAE, RA flares occurred in 42/87 (48%) of cases and IA in 14/203 (7%) of comparators (p<0.0001). Two of the RA flares were grade 3. Pre-existing RA cases were less likely to have rash/dermatitis (5/87 [6%] vs 28/203 [14%], p=0.048), endocrinopathy (2/87 [2%] vs 22/203 [11%], p=0.0078), colitis/enteritis (6/87 [7%] vs 28/203 [14%], p=0.094), and hepatitis (3/87 [4%] vs 19/203 [9%], p=0.043) as irAE. There were no associations of pre-existing RA with other irAE types, including pneumonitis, myocarditis, and myositis.
For pre-existing RA cases, there were 60 deaths during 1,609 person-months of follow-up (IR of 37.3 per 1000 person-months, Table 3). For comparators, there were 127 deaths during 4,538 person-months of follow-up (IR 28.0 per 1000 person-months). The median survival was 13.5 (IQR 5.6, 26.1) months for pre-existing RA cases and 17.2 (IQR 4.5, 33.2) for comparators. In the co-primary analysis for mortality, pre-existing RA cases had a HR of 1.16 (95%CI 0.87-1.57), adjusted for pack-years, cancer duration, previous cancer treatment, and CCI. Pre-existing RA cases on baseline DMARDs had adjusted (a)HR for mortality of 1.43 (95%CI 0.94-2.18).
Table 3.
Hazard ratios for mortality after immune checkpoint inhibitor initiation, comparing pre-existing RA cases (n=87) and matched non-RA comparators (n=203).
| Deaths | Person- months |
Incidence rate (95%CI) per 1000 person-months |
Unadjusted HR (95%CI) |
Multivariable* HR (95%CI) |
|
|---|---|---|---|---|---|
| All patients (co-primary analysis for mortality) | |||||
| Pre-existing RA cases | 60 | 1,609 | 37.3 (27.9, 46.7) | 1.22 (0.90, 1.65) | 1.16 (0.86, 1.57) |
| Matched comparators | 127 | 4,538 | 28.0 (23.1, 32.9) | 1.0 (Ref) | 1.0 (Ref) |
| RA cases on baseline DMARDs and their comparators | |||||
| RA cases | 33 | 593 | 55.6 (36.6, 74.6) | 1.66 (1.10, 2.49) | 1.43 (0.94, 2.18) |
| Matched comparators | 58 | 2,175 | 26.7 (19.8, 33.5) | 1.0 (Ref) | 1.0 (Ref) |
| RA cases not on baseline DMARDs and their comparators | |||||
| RA cases | 27 | 1,015 | 26.6 (16.6, 36.6) | 0.90 (0.58, 1.41) | 0.92 (0.59, 1.45) |
| Matched comparators | 69 | 2,363 | 29.2 (22.3, 36.1) | 1.0 (Ref) | 1.0 (Ref) |
In addition to matching factors of age, sex, calendar year, cancer type, target of ICI, adjusted for smoking pack-years, cancer duration, previous chemotherapy or hormonal therapy, previous radiation, previous stem cell transplant/CAR-T, and continuous Charlson Comorbidity Index.
CAR-T, chimeric antigen receptor T cells; CI, confidence interval; DMARDs, disease-modifying antirheumatic drugs; HR, hazard ratio; ICI, immune checkpoint inhibitor.
The median months from ICI initiation to first irAE was 1.8 (IQR 0.7, 7.0) for pre-existing RA cases and 4.7 (IQR 2.0, 15.7) for comparators (Table 4). Pre-existing RA cases had 53 any all-grade irAE outcomes during 583 person-months (IR 90.9 per 1000 person-months). For comparators, there were 99 any all-grade irAE outcomes during 2,475 person-months (IR of 40.0 per 1000 person-months). In the co-primary analysis for irAE risk, pre-existing RA cases had aHR of 1.72 (95%CI 1.20-2.72) for any all-grade irAE vs comparators. For any grade 3+ irAE, pre-existing RA cases had aHR of 1.06 (95%CI 0.53-2.12). Analyses accounting for competing risk of death had similar results. After RA flare/IA events were excluded, pre-existing RA cases had lower risk than comparators for any all-grade irAE (aHR 0.59, 95%CI 0.38-0.92).
Table 4.
Risk of irAE after immune checkpoint inhibition, comparing pre-existing RA cases (n=87) to matched non-RA comparators (n=203).
| irAE cases | Person- months |
Incidence rate (95%CI) per 1000 person- months |
Unadjusted Cox HR (95%CI) |
Adjusted Cox HR (95%CI) |
Unadjusted competing risk sdHR (95%CI) |
Adjusted competing risk sdHR (95%CI) |
|
|---|---|---|---|---|---|---|---|
| Outcome: all-grade irAE (co-primary analysis for irAE) | |||||||
| Pre-existing RA cases | 53 | 583 | 90.9 (66.4, 115.3) | 1.63 (1.15, 2.31) | 1.72 (1.20, 2.47) | 1.85 (1.30, 2.62) | 2.02 (1.41, 2.89) |
| Matched comparators | 99 | 2,475 | 40.0 (32.1, 47.9) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Outcome: grade 3+ irAE | |||||||
| Pre-existing RA cases | 12 | 1,394 | 8.6 (3.7, 13.5) | 0.96 (0.49, 1.88) | 1.06 (0.53, 2.12) | 1.03 (0.52, 2.03) | 1.24 (0.60, 2.53) |
| Matched comparators | 30 | 4,041 | 7.4 (4.8, 10.1) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Outcome: all-grade irAE, excluding RA flare and IA | |||||||
| Pre-existing RA cases | 26 | 1,042 | 25.0 (15.4, 34.5) | 0.56 (0.36, 0.87) | 0.59 (0.38, 0.92) | 0.58 (0.37, 0.92) | 0.62 (0.39, 0.98) |
| Matched comparators | 99 | 2,484 | 39.9 (32.0, 47.7) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Outcome: grade 3+ irAE, excluding RA flare and IA | |||||||
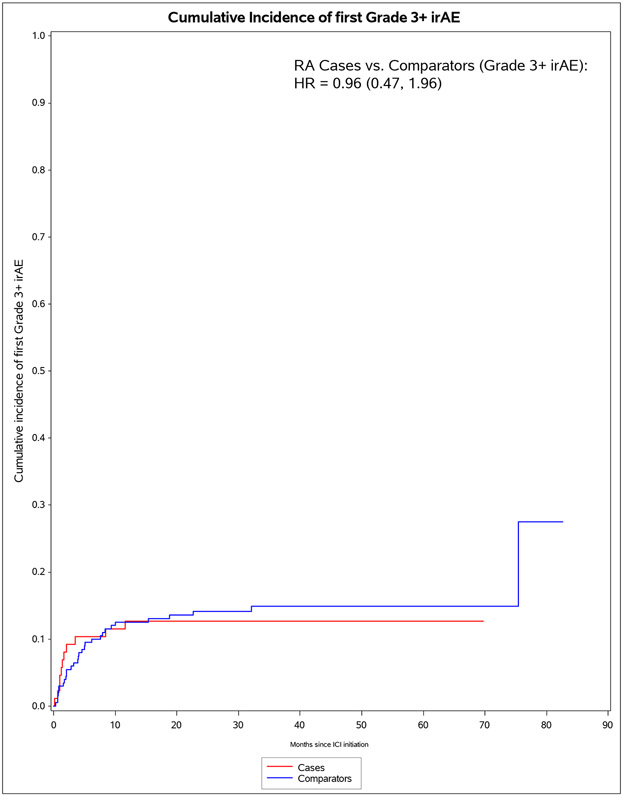
| Pre-existing RA cases | 11 | 1,406 | 7.8 (3.2, 12.5) | 0.87 (0.43, 1.74) | 0.96 (0.47, 1.96) | 0.93 (0.46, 1.87) | 1.11 (0.53, 2.32) |
| Matched comparators | 30 | 4,041 | 7.4 (4.8, 10.1) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
In addition to matching factors of age, calendar year, cancer type, target of ICI, adjusted for smoking pack-years, cancer duration, previous chemotherapy or hormonal therapy, previous radiation, previous stem cell transplant/CAR-T, and continuous Charlson comorbidity index.
CAR-T, chimeric antigen receptor T cells; CI, confidence interval; HR, hazard ratio; IA, inflammatory arthritis; ICI, immune checkpoint inhibitor; irAE, immune-related adverse event; RA, rheumatoid arthritis; sdHR, subdistribution hazard ratio.
Post-baseline factors (ICI number and duration; immunosuppressant use), sensitivity, and stratified analyses are shown in the Appendix pp.7-23. In the subgroup of patients with melanoma, pre-existing RA cases had aHR for mortality of 2.12 (95%CI 0.88-5.07). Pre-existing RA patients with moderate/high RA disease activity at baseline had HR of 3.73 (95%CI 1.17-11.87) for irAE while those with remission/low had HR of 1.97 (95%CI 1.29-3.02, Among males, pre-existing RA had aHR for all-grade irAE of 2.78 (95%CI, 1.57-4.93); among females, pre-existing RA cases had aHR for all-grade irAE of 1.56 (95%CI 0.94-2.52, sex/case-comparator p for interaction=0.26).
DISCUSSION
In this retrospective comparative cohort study, outcomes of patients with pre-existing RA who received ICI for cancer treatment had similar mortality as comparators without autoimmune disease. While patients with pre-existing RA patients had higher risk for any all-grade irAE, this was mostly due to RA flares that occurred in nearly half of pre-existing RA cases but were mostly mild. Patients with pre-existing RA were less likely to experience some other types of irAE than comparators, such as dermatitis/rash, endocrinopathy, and hepatitis, suggesting that the immune system may be primed for RA flares or that treatment of RA flares may prevent other irAE types. Pre-existing RA cases had similar severe irAE risk as comparators, and there were no deaths due to irAE among pre-existing RA cases. These results suggest that pre-existing RA should not be considered a contraindication for appropriate ICI treatment for cancer.
Most previous studies on patients with pre-existing autoimmune diseases who received ICI broadly investigated heterogeneous diseases and many had no comparator group and limited ability to account for confounders. Two systematic reviews identified high flare rates of pre-existing autoimmune disease after ICI initiation14,23. We found that nearly half of patients with pre-existing RA flared, usually soon after ICI initiation. However, the RA flares were mostly mild. Another single-center study investigated 16 patients with pre-existing rheumatic diseases (5 with RA)15. A nationwide French study identified 112 patients with pre-existing rheumatic disease (20 with RA) but had no comparator group9. Only one previous study focused on patients with pre-existing RA initiating ICI, but only included 22 patients and had no comparator group16. That study showed that 55% had RA flares, 32% experienced other irAE, and 9% experienced severe irAE16, similar to our findings. Among comparators, we found that ICI-induced inflammatory arthritis occurred in 7% of patients, similar to previous research8.
Some studies investigating pre-existing autoimmune diseases and ICI outcomes included comparator groups. A Dutch study used registry data from 4,367 melanoma patients to identify 415 with pre-existing autoimmune diseases (227 with rheumatic disease)24. They also found no differences in mortality or rates of irAE for those with autoimmune diseases24. Autoimmune patients were more likely to discontinue ICI, and inflammatory bowel disease patients were more likely to develop colitis after ICI24. A French registry study of melanoma patients investigated 110 patients with pre-existing autoimmune disease (only 11 with RA) and 330 matched controls20. Unlike our study, they found that autoimmune patients had improved survival compared to controls, but also higher risk for severe irAE20. This may be explained by their study mostly being composed of autoimmune thyroid disease patients. Another large EHR study found that pre-existing autoimmune diseases had no overall association with mortality, but patients with Hashimoto disease had lower mortality25. Thus, patients with pre-existing autoimmune disease may safely be treated with ICI while monitoring for underlying disease flare and other irAE.
We found that patients with pre-existing RA had lower risk for some types of irAE, particularly rash/dermatitis, endocrinopathy, hepatitis, and colitis. The immune system may be primed for articular flares in patients with pre-existing RA, perhaps due to tissue-resident memory T cells26 or subclinical RA disease activity then triggered by ICI. This predisposition for articular inflammation may spare patients from other irAE types. Potentially lethal irAE had either similar or lower rates in patients with RA. Patients with RA are prone to developing ILD as an extra-articular manifestation27,28. Even though 15% of pre-existing RA had ILD, pneumonitis rates were similar to comparators. While RA patients may develop secondary Sjögren syndrome29, none developed sicca syndrome after ICI30.
Our study has several limitations to consider. We only analyzed patients with pre-existing RA whose oncologists chose to initiate ICI, so we do not have data about patients with RA whose cancer was not treated with ICI perhaps due to active RA or perceived risk of poor outcomes. Relatively few patients in our study had moderate/high RA disease activity at ICI initiation. We may have missed some RA cases with insufficient documentation or who were seen by an outside rheumatologist. However, the RA prevalence (101/10,901 [0.93%]) is similar to population estimates. There were relatively few patients with pre-existing RA on immunosuppression and many had low disease activity/remission at baseline. This may have been explained by preceding chemotherapy that resulted in DMARD discontinuation and also treated RA. Some patients with high RA disease activity may have delayed or deferred ICI due to risk of flare or irAE. Thus, our findings may not apply to all RA patients, particularly those with high disease activity or those whose oncologist chose not to treat with ICI. We were limited in examining whether changes in immunosuppression affected outcomes in this study. Future studies among only pre-existing RA patients are needed to examine immunosuppressive changes and outcomes. We found a suggestion that pre-existing RA cases on baseline immunosuppression may have higher mortality. However, this was not statistically significant. Patients with moderate/high baseline RA disease activity had especially high irAE risk, so these patients should be monitored closely for irAE.
We were dependent upon EHR documentation to identify RA flares and other irAE. Oncologists and rheumatologists may have been monitoring closely for RA flares, and abstractors were not blinded to RA case/comparator status when determining RA flares and irAE types from the EHR. Many RA flares were diagnosed by oncologists rather than rheumatologists, so may have some inaccuracy. Disruptions of ICI after baseline may have mediated associations. However, the number and duration of ICI infusions between pre-existing RA cases and comparators were similar. There may have been missing data on some important confounders/RA characteristics. These include validated measures of RA disease activity, baseline inflammatory markers, RA-related autoantibodies, functional status, tumor markers/genetics such as PD-1 staining, and family history of autoimmunity. We could not adjust for baseline immunosuppression since comparators were not on these medications. It is possible that a larger study may have detected a modestly increased mortality risk for pre-existing RA. We analyzed a single healthcare system, so results may not generalize to other geographic areas with more diversity.
In conclusion, we found that patients with pre-existing RA initiating ICI had similar risk for mortality and severe irAE as matched comparators. While patients with pre-existing RA were more likely to experience irAE, this was mostly due to mild RA flares. Overall, these results suggest that patients with pre-existing RA can safely receive ICI for cancer treatment.
Supplementary Material
Figure 1.
Cumulative incidence curves for outcomes after immune checkpoint inhibitor initiation for cancer treatment for pre-existing rheumatoid arthritis (RA) cases (n=87) and matched non-RA comparators (n=203). A. All-cause mortality. B. All-grade irAE. C. Grade 3+ irAE. D. All-grade irAE, excluding RA flare for cases and inflammatory arthritis for matched comparators. E. Grade 3+ irAE, excluding RA flare for cases and inflammatory arthritis for matched comparators.
RESEARCH IN CONTEXT.
Evidence before this study
We searched Pubmed for articles published in English from database inception to November 11, 2022, using the terms: ((“rheumatoid arthritis” or “pre-existing autoimmune”) AND “immune checkpoint inhibitor)” NOT ((review) OR (editorial) OR “case report”)). We found 248 articles. Most studied de novo inflammatory arthritis occurring as an immune-related adverse event (irAE) after immune checkpoint inhibitor (ICI) initiation for cancer, rather than flares of arthritis in pre-existing rheumatoid arthritis (RA). A 2019 French retrospective cohort study identified 112 patients with pre-existing autoimmune disease that received ICI, but only 20 had pre-existing RA and there were no comparators. A small 2021 study investigated 21 pre-existing RA patients who initiated ICI but focused on RA flare management and did not include comparators. A large retrospective study using TriNetX electronic health records found that pre-existing autoimmune disease was not associated with mortality in patients receiving anti-PD-1 or anti-PD-L1 compared to those without autoimmune diseases but was not focused on RA and did not investigate risk for irAE. A recent prospective study of patients with melanoma initiating ICI found that 110 patients with pre-existing autoimmune diseases had higher risk for all-grade and severe irAE but had improved survival over matched controls without autoimmune disease. However, autoimmune thyroiditis was the most common disease and only 11 had RA in that study. A 2020 meta-analysis summarized the literature of 619 patients with any pre-existing autoimmune disease who received ICI, but the study focused on flare of the underlying disorder rather than on mortality or irAE and did not include comparators.
Added value of this study
This is the largest study to date to focus on patients with a single pre-existing autoimmune condition initiating ICI for cancer that also includes comparators matched to age, sex, year, ICI target, and cancer type/stage. We investigated the clinical outcomes most important to patients and clinicians that include risks for mortality and RA flare as well as irAE presence, type, and severity. We found that patients with pre-existing RA had similar risk for mortality, higher risk for any all-grade irAE (mostly due to RA flares), and similar risk for severe irAE compared with matched comparators. Ours is the first to show that pre-existing RA patients may be at lower risk for some irAE types including dermatitis/rash, endocrinopathy, and hepatitis.
Implications of all the available evidence
Our results showing that pre-existing RA cases had similar risks for mortality and severe irAE as matched comparators suggest that pre-existing RA should not be considered a contraindication for receiving ICI for cancer treatment.
Funding:
Dr. Yoshida was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant numbers K23 AR076453 and R03 AR081309). Dr. MacFarlane is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant number K23 AR080206). Dr. Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant numbers R01 AR077607, P30 AR070253, and P30 AR072577), the Rheumatology Research Foundation (R Bridge Award), the R. Bruce and Joan M. Mickey Research Scholar Fund, the Llura Gund Award for Rheumatoid Arthritis Research and Care, and investigator-initiated grant from Bristol Myers Squibb.
Footnotes
Declaration of interests: Dr. Yoshida has received consulting fees from OM1, Inc. at the time of this work and is now employed by and owns stock options from OM1, Inc unrelated to this work. Dr. Shadick is supported by Mallinckrodt, Lilly, BMS, Amgen, Abbvie, and Aqtual unrelated to this work. Dr. LeBoeuf receives consulting fees from Bayer, Seattle Genetics, Sanofi, Silverback, and Synox Therapeutics unrelated to this work. Dr. Buchbinder has received a research grant from Genentech and has participated on advisory boards for Merck, Bristol Myers Squibb, and Novartis unrelated to this work. Dr. Gedmintas has received honoraria for invited lectures for the Harvard Continuing Medical Education programs Intensive Review of Internal Medicine and Innovations and New Practices in Internal Medicine (not sponsored by for-profit institutions). Dr. Gravallese receives royalties from UpToDate and the Rheumatology textbook (published by Elsevier), has received honoraria for scientific lectures at academic institutions/meetings (none for industry or for-profit institutions) and the American College of Rheumatology (serving on task forces for COVID treatment and vaccine guidelines), has received reimbursement for travel to academic institutions and meetings hosted by the American College of Rheumatology, and has received partial salary support serving as president-elect and president of the American College of Rheumatology and as an Associate Editor for the New England Journal of Medicine. Dr. Sparks has received research support from Bristol Myers Squibb and performed consultancy for AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer unrelated to this work. All other authors report no conflicts of interest.
Data sharing:
Data are available upon request to the corresponding author with appropriate institutional review board approval.
REFERENCES
- 1.Doroshow DB, Sanmamed MF, Hastings K, et al. Immunotherapy in Non-Small Cell Lung Cancer: Facts and Hopes. Clin Cancer Res 2019; 25(15): 4592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pasquali S, Hadjinicolaou AV, Chiarion Sileni V, Rossi CR, Mocellin S. Systemic treatments for metastatic cutaneous melanoma. Cochrane Database Syst Rev 2018; 2(2): CD011123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang C, Qiao W, Jiang Y, et al. The landscape of immune checkpoint inhibitor plus chemotherapy versus immunotherapy for advanced non-small-cell lung cancer: A systematic review and meta-analysis. J Cell Physiol 2020; 235(5): 4913–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang R, Zhu J, Liu Y, et al. Efficacy of immune checkpoint inhibitors in the treatment of non-small cell lung cancer patients with different genes mutation: A meta-analysis. Medicine (Baltimore) 2021; 100(10): e19713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cunningham-Bussel A, Wang J, Prisco LC, et al. Predictors of Rheumatic Immune-Related Adverse Events and De Novo Inflammatory Arthritis After Immune Checkpoint Inhibitor Treatment for Cancer. Arthritis Rheumatol 2022; 74(3): 527–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev 2016; 44: 51–60. [DOI] [PubMed] [Google Scholar]
- 7.Chan KK, Bass AR. Monitoring and Management of the Patient with Immune Checkpoint Inhibitor-Induced Inflammatory Arthritis: Current Perspectives. J Inflamm Res 2022; 15: 3105–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh N, Tiongson MD, Stewart C, et al. Checkpoint Inhibitor-Associated Arthritis: A Systematic Review of Case Reports and Case Series. J Clin Rheumatol 2021; 27(8): e317–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tison A, Quere G, Misery L, et al. Safety and Efficacy of Immune Checkpoint Inhibitors in Patients With Cancer and Preexisting Autoimmune Disease: A Nationwide, Multicenter Cohort Study. Arthritis Rheumatol 2019; 71(12): 2100–11. [DOI] [PubMed] [Google Scholar]
- 10.Giat E, Ehrenfeld M, Shoenfeld Y. Cancer and autoimmune diseases. Autoimmun Rev 2017; 16(10): 1049–57. [DOI] [PubMed] [Google Scholar]
- 11.Khan SA, Pruitt SL, Xuan L, Gerber DE. Prevalence of Autoimmune Disease Among Patients With Lung Cancer: Implications for Immunotherapy Treatment Options. JAMA Oncol 2016; 2(11): 1507–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez-Ruiz E, Minute L, Otano I, et al. Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature 2019; 569(7756): 428–32. [DOI] [PubMed] [Google Scholar]
- 13.Verheijden RJ, May AM, Blank CU, et al. Association of Anti-TNF with Decreased Survival in Steroid Refractory Ipilimumab and Anti-PD1-Treated Patients in the Dutch Melanoma Treatment Registry. Clin Cancer Res 2020; 26(9): 2268–74. [DOI] [PubMed] [Google Scholar]
- 14.Abdel-Wahab N, Shah M, Lopez-Olivo MA, Suarez-Almazor ME. Use of Immune Checkpoint Inhibitors in the Treatment of Patients With Cancer and Preexisting Autoimmune Disease: A Systematic Review. Ann Intern Med 2018; 168(2): 121–30. [DOI] [PubMed] [Google Scholar]
- 15.Richter MD, Pinkston O, Kottschade LA, Finnes HD, Markovic SN, Thanarajasingam U. Brief Report: Cancer Immunotherapy in Patients With Preexisting Rheumatic Disease: The Mayo Clinic Experience. Arthritis Rheumatol 2018; 70(3): 356–60. [DOI] [PubMed] [Google Scholar]
- 16.Efuni E, Cytryn S, Boland P, et al. Risk of Toxicity After Initiating Immune Checkpoint Inhibitor Treatment in Patients With Rheumatoid Arthritis. J Clin Rheumatol 2021; 27(7): 267–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010; 62(9): 2569–81. [DOI] [PubMed] [Google Scholar]
- 18.England BR, Tiong BK, Bergman MJ, et al. 2019 Update of the American College of Rheumatology Recommended Rheumatoid Arthritis Disease Activity Measures. Arthritis Care Res (Hoboken) 2019; 71(12): 1540–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bongartz T, Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum 2010; 62(6): 1583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Placais L, Dalle S, Dereure O, et al. Risk of irAEs in patients with autoimmune diseases treated by immune checkpoint inhibitors for stage III or IV melanoma: results from a matched case-control study. Ann Rheum Dis 2022; 81(10): 1445–52. [DOI] [PubMed] [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40(5): 373–83. [DOI] [PubMed] [Google Scholar]
- 22.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Amer Statist Assoc. 94.446 (1999): 496–509. [Google Scholar]
- 23.Xie W, Huang H, Xiao S, Fan Y, Deng X, Zhang Z. Immune checkpoint inhibitors therapies in patients with cancer and preexisting autoimmune diseases: A meta-analysis of observational studies. Autoimmun Rev 2020; 19(12): 102687. [DOI] [PubMed] [Google Scholar]
- 24.van der Kooij MK, Suijkerbuijk KPM, Aarts MJB, et al. Safety and Efficacy of Checkpoint Inhibition in Patients With Melanoma and Preexisting Autoimmune Disease : A Cohort Study. Ann Intern Med 2021; 174(5): 641–8. [DOI] [PubMed] [Google Scholar]
- 25.Tang K, Tiu BC, Wan G, et al. Pre-Existing Autoimmune Disease and Mortality in Patients Treated with Anti-PD-1 and Anti-PD-L1 Therapy. J Natl Cancer Inst 2022; 114(8): 1200–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang MH, Levescot A, Nelson-Maney N, et al. Arthritis flares mediated by tissue-resident memory T cells in the joint. Cell Rep 2021; 37(4): 109902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sparks JA, Jin Y, Cho SK, et al. Prevalence, incidence and cause-specific mortality of rheumatoid arthritis-associated interstitial lung disease among older rheumatoid arthritis patients. Rheumatology (Oxford) 2021; 60(8): 3689–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kronzer VL, Huang W, Dellaripa PF, et al. Lifestyle and Clinical Risk Factors for Incident Rheumatoid Arthritis-associated Interstitial Lung Disease. J Rheumatol 2021; 48(5): 656–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown LE, Frits ML, Iannaccone CK, Weinblatt ME, Shadick NA, Liao KP. Clinical characteristics of RA patients with secondary SS and association with joint damage. Rheumatology (Oxford) 2015; 54(5): 816–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cappelli LC, Gutierrez AK, Bingham CO 3rd, Shah AA. Rheumatic and Musculoskeletal Immune-Related Adverse Events Due to Immune Checkpoint Inhibitors: A Systematic Review of the Literature. Arthritis Care Res (Hoboken) 2017; 69(11): 1751–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request to the corresponding author with appropriate institutional review board approval.