Abstract
Background:
The overlapping biomechanical relationship between the lumbosacral spine and pelvis poses unique challenges to patients with concomitant pathologies limiting spinopelvic range of motion.
Purpose:
To assess the influence of concomitant, symptomatic lumbosacral spine pathology on patient-reported outcome measures (PROMs) after hip arthroscopy for the treatment of femoroacetabular impingement (FAI) and symptomatic labral tears.
Study Design:
Cohort study; Level of evidence, 3.
Methods:
A retrospective query of prospectively collected data identified patients aged ≥18 years with a minimum 24-month follow-up who underwent hip arthroscopy by a single surgeon for the treatment of symptomatic labral tears secondary to FAI. Patients were stratified into cohorts based on the presence (hip-spine [HS]) or absence (matched control [MC]) of symptomatic lumbosacral spine pathology. Inclusion within the HS cohort required confirmation of lower back pain/symptoms on preoperative surveys plus a diagnosis of lumbosacral spine pathology verified by radiology reports and correlating clinical documentation. Patients with previous spine surgery were excluded. PROMs were compared between groups, along with rates of achieving minimal clinically important difference (MCID) thresholds, Patient Acceptable Symptom State (PASS) thresholds, revision arthroscopy, and conversion to total hip arthroplasty (THA).
Results:
A total of 70 patients with lumbosacral pathology were coarsened exact matched to 87 control patients without spinal pathology. The HS cohort had preoperative baseline scores that were significantly worse for nearly all PROMs. Follow-ups at 3, 6, 12, and 24 months displayed similar trends, with the HS cohort demonstrating significantly worse scores for most collected outcomes. However, at every time point, HS and MC patients exhibited similar magnitudes of improvement across all PROM and pain metrics. Furthermore, while significantly fewer HS patients achieved PASS for nearly all PROMs at 12- and 24-month follow-ups, MCID thresholds were reached at similar or greater rates across all PROMs relative to the MC cohort. Finally, there were no significant differences in rates of revision or THA between cohorts at maximum available follow-up.
Conclusion:
After hip arthroscopy to address labral tears in the setting of FAI, patients with symptomatic lumbosacral pathologies and no history of spine surgery were found to exhibit inferior pre- and postoperative PROMs but achieved statistically similar clinical benefit and rates of PROM improvement through 24-month follow-up compared with the MC cohort with isolated hip disease. These findings aid in providing a realistic recovery timeline and evidence that coexisting hip and spine disorders are not a contraindication for arthroscopic hip preservation surgery.
Keywords: hip-spine syndrome, femoroacetabular impingement, hip arthroscopy, lumbosacral spine disease
For patients with untreated femoroacetabular impingement (FAI) syndrome, variations in regional loading across the acetabulum and femoral head pose an elevated risk for the early progression of degenerative joint disease. 46 The deleterious effect of cam and pincer lesions on the labrum, chondrolabral junction, and articular cartilage may subsequently interfere with activities of daily living and warrant the need for early conversion to total hip arthroplasty (THA). 52 Numerous studies have highlighted the benefits of arthroscopic labral repair with femoroacetabular osteoplasties in improving hip symptoms and providing favorable long-term outcomes. 34 However, for a subset of patients with concomitant lumbosacral spine disease and overlapping hip symptomatology, surgeons may be challenged in isolating the predominant cause of pain and identifying appropriate surgical candidates. 50
Hip-spine (HS) syndrome was originally described by Offierski and MacNab 40 in reference to concurrent hip osteoarthritis and lumbar spine degenerative disease commonly seen in the elderly population. However, since that time, investigations into the biomechanical relationship between the lumbar spine, pelvis, and hip have revealed that alterations in spinopelvic motion may have negative implications for younger patients with limited evidence of osteoarthritis. 50 Secondary HS syndrome can affect any age demographic and largely stems from the tandem of compensatory stresses from either structure that exacerbates symptoms in the other. 50 In the early stages of FAI, studies indicate that lumbosacral mobility may be crucial to maintain sagittal balance and counteract the restricted range of motion (ROM) through the hip.13,23,50 However, over time, compensatory variations in lumbar lordosis, pelvic incidence, pelvic tilt, and sacral slope can accelerate arthrosis in facet joints, leading to lower back pain, stiffness, and possible lumbar stenosis.13,15,50 Ultimately, loss of spinopelvic mobility may exacerbate hip-specific symptoms in patients with FAI, as activities requiring high degrees of hip flexion (eg, sitting, squatting) inherently perpetuate mechanical injury to the chondrolabral complex.13,15
The link between spinopelvic mobility and pelvic sagittal alignment has been well-documented in THA literature, where extremes of pelvic alignment, lumbar disease, previous fusion surgery, and stiffness have been associated with impingement, dislocation, early failure, and need for revision surgery.11-13,23,26 While a thorough preoperative workup and optimal implant selection/positioning may mitigate adverse events after THA, literature reporting outcomes in the setting of hip preservation remains limited.4,6,14,18,26 The true incidence of secondary HS syndrome in patients with limited hip arthritis has yet to be determined; however, studies suggest that the prevalence of low back pain may be as high as 60% in the setting of FAI. 7 As such, there is a clinical need to further assess the effect of coexisting lumbosacral spine pathology for patients seeking surgical treatment for FAI. Three recent systematic reviews have largely surmised that patients with FAI who have lumbosacral pathologies experience improved but inferior outcomes after hip arthroscopy compared with controls without concomitant spinal conditions.1,15,25 However, secondary analyses continue to be limited given the paucity of available studies that use heterogeneous patient populations, variable outcome metrics, and short lengths of follow-up.1,4,15,25,43
The purpose of this present study was to conduct a matched, case-control analysis to assess the influence of symptomatic lumbosacral pathology on hip arthroscopy outcomes for patients with FAI and symptomatic labral tears. Borrowing from available literature, we hypothesized that preexisting lumbosacral spine pathology would adversely affect patient-reported outcome measures (PROMs), rates of achieving clinically significant postoperative outcome thresholds (eg, minimal clinically important difference [MCID] and Patient Acceptable Symptom State [PASS]), need for revision arthroscopy, and conversion to THA.
Methods
Study Population
During the study period, the senior author’s (S.D.M.) standardized, preoperative evaluation for patients presenting to clinic with hip pain consisted of hip and pelvis radiographs (ie, anteroposterior pelvic and Dunn lateral views) and a physical examination with provocation testing of the labrum and assessment for impingement-related symptoms (eg, pain and/or limited ROM with flexion, adduction, and internal rotation or flexion, abduction, and external rotation).17,33 Patients with positive clinical and radiographic findings underwent magnetic resonance arthrography to evaluate for labral pathologies coupled with a diagnostic and therapeutic intra-articular hip joint injection (eg, combined local anesthetic with a low-dose corticosteroid). 27 Finally, all patients trialed nonoperative management, including formal physical therapy, for a minimum of 3 months. Patients who had unsuccessful nonoperative management and consented to undergo hip arthroscopy were offered enrollment in this study. 41 This study was approved by our institutional review board (No. 2019P002191/No. 2013P001442).
Study Design
Data were prospectively collected for enrolled patients undergoing hip arthroscopy by a single, fellowship-trained surgeon (S.D.M.) and retrospectively reviewed. Patient inclusion criteria consisted of the following: (1) age ≥18 years; (2) primary hip arthroscopy for the treatment of symptomatic labral tears secondary to FAI between May 2014 and December 2020; (3) complete preoperative, baseline PROMs; and (4) follow-up PROMs for ≥1 time point at ≥24 months (ie, 24 months, 3 years, and/or 5 years) postoperatively. Exclusion criteria consisted of labral debridement, previous surgery on the ipsilateral hip/leg, previous spine surgery (eg, decompression laminectomy/discectomy, kyphoplasty, fusion), radiographic evidence of hip dysplasia (lateral center-edge angle [LCEA] <20°), advanced hip osteoarthritis (eg, Tönnis grade >1), and/or previous hip conditions (eg, fracture, inflammatory arthropathies, Legg-Calve-Perthes disease, slipped capital femoral epiphysis, avascular necrosis).
A review of our institution’s electronic medical record (EMR) and prospectively collected data allowed for patients to be stratified into cohorts based on the presence or absence of concomitant symptomatic lumbosacral spinal pathology (ie, HS cohort vs matched control [MC] cohort). Placement into the HS cohort required (1) confirmation of lower back pain/symptoms on preoperative baseline survey questionnaires, (2) preoperative imaging with a pathologic lumbosacral spine diagnosis confirmed by the radiologist, and (3) correlating clinical documentation by the physician who either ordered imaging or initially worked up the patient for lower back symptoms. 18 Conversely, patients were placed in the MC cohort based on (1) denial of lower back pain/symptoms on preoperative, baseline survey questionnaires prior to hip arthroscopy and (2) no clinical documentation or imaging corroborating the diagnosis of a back condition. Importantly, patients in both cohorts denied receipt of spine surgery involving any level preoperatively, and the EMR was also queried to verify the absence of any documented spine interventions. When possible, discrepancies in patient survey responses were reconciled if sufficient EMR documentation was available following verification/agreement by 2 authors (S.D.M. and N.J.C.). Eligible patients in the HS cohort were matched to patients in the MC cohort using coarsened exact matching (CEM) with bins defined on the basis of age <34, ≥34 to <50, or ≥50 years; male or female sex; and body mass index (BMI) of <24, ≥24 to <30, or ≥30. CEM was employed over other matching techniques based on previous literature citing its ability to reduce imbalance within observational data when a few strong confounders must be controlled.16,20,44
Abbreviated Surgical Technique
After the administration of general anesthesia, all patients were positioned supine on a hip distraction table with a gel-padded perineal post. Intra-articular access was facilitated via a puncture capsulotomy technique to avoid biomechanical disruption of the iliofemoral ligament and zona orbicularis. Under fluoroscopic guidance, the anterolateral portal was first established using intra-articular fluid distention.2,10,24 Next, under direct arthroscopic visualization, anterior and midanterior portals were established, followed by a Dienst portal (placed one-third the distance between the anterior superior iliac spine and the anterolateral portal). 10 A thorough diagnostic survey was performed to assess the extent of damage to the labrum, chondrolabral junction, and cartilage surfaces. As previously published, the senior surgeon’s (S.D.M.) hip arthroscopy technique includes sparing use of intermittent traction, pulsed intra-articular lavage to maintain ambient intra-articular temperatures, and an emphasis on chondrolabral junction preservation.31,36,38,47,49
As clinically indicated, acetabular recession and acetabuloplasty were carried out to address underlying pincer deformities without violating the chondrolabral junction. 49 For labral lesions, repair was performed if adequate, healthy tissue was amenable to suture anchor fixation. Conversely, labral reconstruction was employed via a published capsular augmentation technique if the labrum was found to be irreparable (eg, tissue insufficiency, advanced degeneration, complex tears).22,36,38 Of note, within the study period, the senior surgeon transitioned from the use of microfracture to a standardized method of bone marrow aspirate concentrate (BMAC) augmentation to address full-thickness chondral flaps, focal Outerbridge grade ≥2 lesions, and/or chondrolabral junction breakdown.28,29 As such, operative notes were queried to ascertain which patients received microfracture versus BMAC augmentation. Importantly, no other substantial changes or variations in surgical technique were applied by the senior author within the study period. After addressing pincer impingement, labral defects, and full-thickness chondral lesions, traction was released to ensure restoration of the hip suction seal and confirm an in-round labral repair. 47 As appropriate, osseous cam deformities were resected via femoroplasty while the hip was flexed to approximately 45°. Last, a dynamic ROM examination ensured the adequacy of femoroacetabular decompression and was followed by the closure of all incisions.
Postoperative Rehabilitation
Postoperatively, all patients were subjected to the same patient-guided rehabilitation program and prescribed daily deep vein thrombosis prophylaxis (aspirin, 81 mg) for 3 weeks. Patients were permitted immediate weightbearing as tolerated using a flat-foot gait with crutches. At 6 weeks postoperatively, patients began the use of a stationary bicycle with minimal resistance. At 10 weeks, clearance was given to use an elliptical trainer on low resistance or swimming (± buoy board) while avoiding intense flutter kicks. At 4 months, patients resumed light strengthening exercises, including short arc leg presses and hamstring curls. Finally, at 6 months postoperatively, patients were permitted to resume impact-loading activities as tolerated. 35
Data Collection and Functional Outcome Evaluation
Demographic and descriptive data were collected preoperatively, including age, sex, laterality, BMI, LCEA, Tönnis angle, α angle, type of FAI, and radiographic Tönnis classification. Intraoperative variables of interest included arthroscopic procedures performed, presence of full-thickness chondral lesions/flap, and degree of injury to the labrum/chondrolabral junction based on validated classification scales.5,30
Survey questionnaires were distributed to patients before surgery (baseline); postoperatively at 3, 6, and 12 months; and annually thereafter. Hip-specific PROMs included modified Harris Hip Score (mHHS), Hip Outcome Score–Activities of Daily Living (HOS-ADL), Hip Outcome Score–Sports Subscale (HOS-SS), Non-Arthritic Hip Score (NAHS), and the International Hip Outcome Tool–33 (iHOT-33). Pain symptoms were tracked over the study period using a standard visual analog scale (VAS). Clinically meaningful outcomes were assessed by calculating the percentage of patients who achieved threshold PROM scores for MCID and PASS, as defined by Nwachukwu et al 37 and Rosinsky et al. 45 Finally, patient satisfaction, rates of revision hip arthroscopy, and the incidence of conversion to THA were also tracked via survey responses and review of the EMR.
A Priori Power Analysis and Statistical Analysis
Based on previous research evaluating the association between low back pain and hip function after hip arthroscopy, an a priori power analysis was performed using an estimated standard deviation in iHOT-33 scores of 17.7 and the corresponding 5-year MCID threshold value of 15.1.6,37 In total, 21 and 25 patients were needed in the HS and MC cohorts, respectively, to achieve 80% power.
After CEM, Shapiro-Wilk tests and F tests were performed to determine if continuous data followed normal distributions and demonstrated equal variances, respectively. Categorical variables were analyzed with chi-square or Fisher exact tests, as appropriate. Continuous variables collected at a single time point (eg, age) were compared using 2-tailed independent t tests, while those collected longitudinally (ie, improvement in PROMs/pain scores) were assessed using linear mixed-effects models. 3 The latter provides greater statistical power by linking observations for each participant, accounting for variability between patients, and incorporating all available data rather than excluding participants missing a single follow-up time point, as would be the case in a t test.42,48 Each regression clustered observations at the patient level; modeled time, the presence of symptomatic lumbosacral spine pathology, and their interaction as fixed effects; and included random by-participant intercepts. Time was modeled as a continuous variable to compute weighted mean differences in the improvement of outcome scores across the 24-month study period or as a categorical variable to compare PROMs at discrete time points. Last, a subgroup analysis including HSsub and MCsub patients with a minimum 3-year follow-up was performed to preliminarily compare midterm outcomes at 3 and 5 years. Parameter estimates and descriptive statistics for continuous variables are presented with 95% CIs, with the MC cohort treated as the reference group (if applicable). Frequency statistics are reported for all noncontinuous variables. A P value <.05 was considered statistically significant. Statistical analyses were performed using R Version 4.2.1 (R Foundation for Statistical Computing).
Results
Patient Characteristics
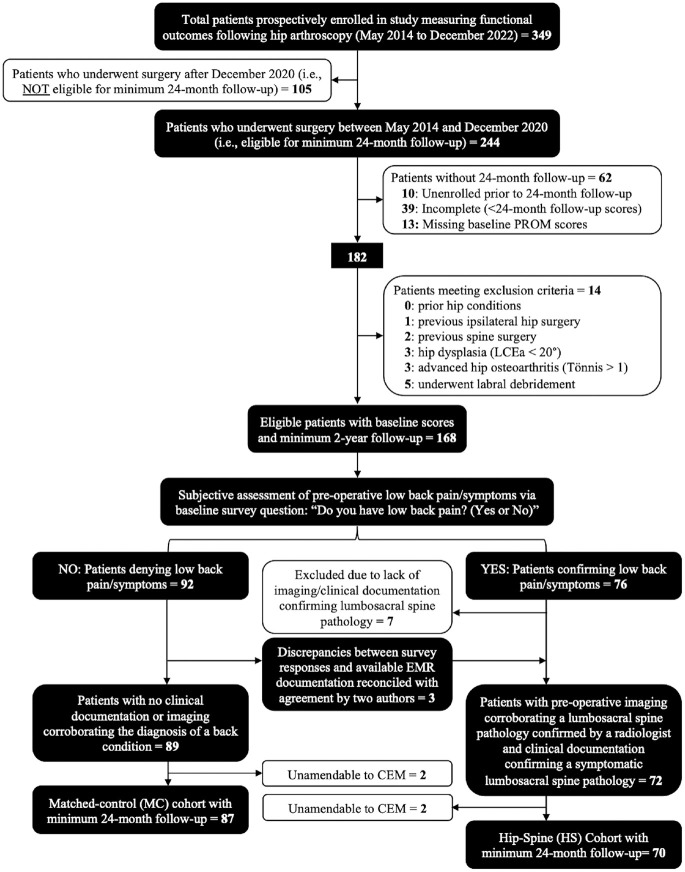
Of the 349 patients prospectively enrolled in this study, 244 underwent primary hip arthroscopy for the treatment of symptomatic labral tears secondary to FAI between May 2014 and December 2020. After applying eligibility criteria and performing CEM, 157 (64%) of these 244 patients were included and are the basis of this report. A total of 70 patients were allocated to the HS cohort and 87 to the MC cohort (Figure 1). No significant differences in patient characteristics were identified between HS and MC cohorts, including sex (53% vs 46% female; P = .391) or BMI (mean [95% CI], 26.1 [25.2-27.0] vs 25.1 [24.2-25.9]; P = .116), however, a trend toward a significant difference was noted in age (39.4 [37.1-41.7] vs 35.8 [33.2-38.4]; P = .051). Additional demographic and preoperative variables are listed in Table 1. For the HS cohort, a review of clinical documentation plus pertinent lumbosacral imaging (ie, radiographs, magnetic resonance, and/or computed tomography imaging) allowed for the classification of lower back pathologies (Table 1). The mean (95% CI) lengths of follow-up were 38.2 (34.9-41.6) and 39.2 (36.1-42.4) months for the HS and MC cohorts, respectively, and 38.8 (36.6-41.1) months for all patients (N = 157) included in the primary analysis (Table 2).
Figure 1.
Flowchart detailing eligibility/selection criteria of patients with minimum 24-month follow-up. PROM, patient-reported outcome metric; LCEa, lateral center edge angle; CEM, coarsened-exact matching.
Table 1.
Patient Characteristics by Cohort a
| Characteristic | HS Cohort (n = 70) | MC Cohort (n = 87) | P Value |
|---|---|---|---|
| Age, mean (95% CI), y | 39.4 (37.1-41.7) | 35.8 (33.2-38.4) | .051 |
| Sex | .391 | ||
| Male | 33 (47.1) | 47 (54.0) | |
| Female | 37 (52.9) | 40 (46.0) | |
| BMI, mean (95% CI) | 26.1 (25.2-27.0) | 25.1 (24.2-25.9) | .116 |
| Laterality | .541 | ||
| Left | 34 (48.6) | 38 (43.7) | |
| Right | 36 (51.4) | 49 (56.3) | |
| α Angle, mean (95% CI), deg | 52.2 (48.9-55.5) | 55.2 (51.7-58.6) | .235 |
| LCEA, mean (95% CI), deg | 37.0 (35.6-38.5) | 37.2 (36.1-38.2) | .894 |
| Tönnis angle, mean (95% CI), deg | 6.7 (5.6-7.9) | 5.9 (4.7-7.1) | .338 |
| Tönnis grade | .915 | ||
| 0 | 36 (51.4) | 44 (50.6) | |
| 1 | 34 (48.6) | 43 (49.4) | |
| Type of FAI | .342 | ||
| Pincer deformity | 41 (58.6) | 42 (48.3) | |
| Cam deformity | 1 (1.4) | 4 (5.6) | |
| Cam and pincer | 28 (40.0) | 41 (47.1) | |
| Lumbar spine pathology | |||
| Degenerative disk disease/spondylosis | 32 (45.7) | — | |
| Degenerative scoliosis | 8 (11.4) | — | |
| Lumbar disc herniation | 12 (17.1) | — | |
| Foraminal stenosis | 12 (17.1) | — | |
| Spondylolysis | 2 (2.8) | — | |
| Spondylolisthesis | 4 (5.7) | — |
Values are presented as number (%) unless otherwise indicated. BMI, body mass index; FAI, femoroacetabular impingement; HS, hip-spine; LCEA, lateral center-edge angle; MC, matched control. Dashes indicate not applicable.
Table 2.
Total and Maximum Available Patient Follow-up at Each Time Point a
| Hip-Spine Cohort (n = 70) | Matched Control Cohort (n = 87) | Total Study Sample (N = 157) | ||||
|---|---|---|---|---|---|---|
| Time Point | Total Available Follow-up | Maximum Available Follow-up for Each Patient | Total Available Follow-up | Maximum Available Follow-up for Each Patient | Total Available Follow-up | Maximum Available Follow-up for Each Patient |
| Baseline | 70 (100) | — | 87 (100) | — | 157 (100) | — |
| 24 Months | 66 (94.3) | 30 (42.9) | 77 (88.5) | 38 (43.7) | 143 (91.1) | 68 (43.3) |
| 3 Years | 28 (40.0) | 19 (27.1) | 31 (35.6) | 18 (20.7) | 59 (37.6) | 37 (23.6) |
| 5 Years | 21 (30.0) | 21 (30.0) | 31 (35.6) | 31 (35.6) | 52 (33.1) | 52 (33.1) |
| Total | 70 (100) | 87 (100) | 157 (100) | |||
Values are presented as number (%) of patients. Dashes indicate not applicable.
Intraoperative Parameters
Intraoperative findings between groups were not significantly different with regard to labral condition (P = .561), focal femoral/acetabular chondral defect severity (eg, Outerbridge grade) (P = .879), chondrolabral junction breakdown (P = .218), and presence of chondral flaps (P = .686). Furthermore, the arthroscopic procedures performed had similar distributions between groups in terms of chondral injury treatment (microfracture vs BMAC vs none; P = .267), labral management (repair vs capsular autograft reconstruction; P = .322), and osteoplasty type (femoroplasty vs acetabuloplasty vs both; P = .342) (Table 3).
Table 3.
Intraoperative Findings and Procedures Performed by Cohort a
| Characteristic | HS Cohort (n = 70) | MC Cohort (n = 87) | P |
|---|---|---|---|
| Labral condition | .561 | ||
| Normal | 00 (0) | 00 (0) | |
| Degeneration | 18 (25.7) | 29 (33.3) | |
| Full-thickness tear | 36 (51.4) | 41 (47.1) | |
| Detachment | 15 (21.4) | 17 (19.5) | |
| Ossification | 1 (1.4) | 00 (0) | |
| Outerbridge grade | .879 | ||
| 0 | 00 (0) | 00 (0) | |
| 1 | 3 (4.3) | 4 (4.6) | |
| 2 | 18 (25.7) | 25 (28.7) | |
| 3 | 36 (51.4) | 46 (52.9) | |
| 4 | 13 (18.6) | 12 (13.7) | |
| Chondrolabral junction | .218 | ||
| Normal | 3 (4.3) | 2 (2.3) | |
| Malacia | 4 (5.7) | 4 (4.6) | |
| Debonding | 11 (15.7) | 7 (8.0) | |
| Cleavage | 31 (44.3) | 54 (62.2) | |
| Defect | 21 (30.0) | 20 (23.0) | |
| Chondral flap | 22 (31.4) | 30 (34.5) | .686 |
| Chondral treatment | .267 | ||
| None | 31 (44.3) | 30 (34.5) | |
| BMAC augmentation | 39 (55.7) | 55 (63.2) | |
| Microfracture | 00 (0) | 2 (2.3) | |
| Labral management | .322 | ||
| Repair alone | 8 (11.4) | 6 (6.9) | |
| Reconstruction with capsular augmentation | 62 (86.6) | 81 (93.1) | |
| Osteoplasty performed | .342 | ||
| Acetabuloplasty | 41 (58.6) | 42 (48.3) | |
| Femoroplasty | 1 (1.4) | 4 (4.6) | |
| Femoroacetabular osteoplasty | 28 (40.0) | 41 (47.1) |
Values are presented as number (%) of patients. BMAC, bone marrow aspirate concentrate; HS, hip-spine; MC, matched control.
Patient-Reported Outcome Scores
When comparing mean PROM and VAS pain scores between groups, patients in the HS cohort displayed significantly worse mean preoperative scores for nearly all outcomes (Table 4). These differences persisted at all follow-ups through 24 months, as the HS cohort demonstrated inferior PROM and VAS pain scores for nearly all collected outcomes, with the exception of the HOS-SS at 3, 6, and 24 months (Table 4). However, when using linear mixed-effects models incorporating time as a categorical variable, HS and MC patients exhibited statistically similar magnitudes of improvement in all functional and pain metrics from baseline to 24 months (Table 5).
Table 4.
Mean PROM/Pain Scores and Interval Improvements at Baseline Through 24-Month Follow-up a
| HS Cohort (n = 70) | MC Cohort (n = 87) | Significance | ||||
|---|---|---|---|---|---|---|
| Characteristic | Mean | Improvement b | Mean | Improvement b | P mean | P improvement |
| Preoperative | ||||||
| mHHS | 59.2 (56.1 to 62.3) | — | 64.2 (61.4 to 67.0) | — | .020 c | — |
| HOS-ADL | 70.1 (67.0 to 73.1) | — | 73.3 (70.6 to 76.1) | — | .119 | — |
| HOS-SS | 38.6 (32.7 to 44.4) | — | 45.6 (40.3 to 50.8) | — | .080 | — |
| NAHS | 60.2 (57.1 to 63.3) | — | 69.0 (66.3 to 71.8) | — | <.001 c | — |
| iHOT-33 | 35.5 (31.1 to 39.9) | — | 45.7 (41.7 to 49.6) | — | <.001 c | — |
| VAS | 5.8 (5.4 to 6.3) | — | 4.5 (4.1 to 5.0) | — | <.001 c | — |
| 3 Months | ||||||
| mHHS | 72.0 (68.7 to 75.4) | 13.0 (9.0 to 17.0) | 80.8 (77.9 to 83.7) | 16.5 (12.9 to 20.0) | <.001 c | .207 |
| HOS-ADL | 78.9 (75.6 to 82.1) | 8.8 (4.4 to 13.2) | 83.6 (80.7 to 86.5) | 10.2 (6.3 to 14.2) | .033 c | .632 |
| HOS-SS | 43.1 (36.9 to 49.3) | 4.8 (–2.3 to 12.0) | 42.9 (37.3 to 48.5) | −2.5 (–9.0 to 3.9) | .971 | .134 |
| NAHS | 73.6 (70.4 to 76.9) | 13.7 (9.5 to 17.8) | 78.8 (75.9 to 81.7) | 9.8 (6.1 to 13.6) | .020 c | .184 |
| iHOT-33 | 57.1 (52.5 to 61.8) | 22.0 (16.2 to 27.7) | 64.1 (59.9 to 68.2) | 19.1 (14.0 to 24.2) | .031 c | .461 |
| VAS | 3.2 (2.7 to 3.7) | −2.9 (−3.6 to −2.2) | 1.8 (1.3 to 2.2) | −2.8 (−3.4 to −2.1) | <.001 c | .833 |
| 6 Months | ||||||
| mHHS | 77.3 (74.0 to 80.6) | 18.1 (14.1 to 22.0) | 83.3 (80.3 to 86.3) | 18.9 (15.4 to 22.5) | .008 c | .752 |
| HOS-ADL | 83.5 (80.2 to 86.7) | 13.3 (8.9 to 17.7) | 88.2 (85.3 to 91.1) | 15.0 (11.1 to 19.0) | .033 c | .561 |
| HOS-SS | 59.9 (53.8 to 66.1) | 21.5 (14.4 to 28.7) | 65.2 (59.7 to 70.7) | 19.8 (13.4 to 26.2) | .212 | .714 |
| NAHS | 80.3 (77.1 to 83.6) | 20.2 (16.0 to 24.4) | 85.8 (82.9 to 88.7) | 16.9 (13.1 to 20.6) | .014 c | .246 |
| iHOT-33 | 64.9 (60.2 to 69.6) | 29.5 (23.8 to 35.2) | 73.7 (69.5 to 77.9) | 28.8 (23.6 to 33.9) | .006 c | .860 |
| VAS | 3.0 (2.5 to 3.5) | −2.9 (−3.6 to −2.2) | 1.6 (1.1 to 2.1) | −2.9 (−3.5 to −2.3) | <.001 c | .938 |
| 12 Months | ||||||
| mHHS | 83.9 (80.7 to 87.1) | 24.5 (20.6 to 28.4) | 88.8 (85.8 to 91.7) | 24.3 (20.7 to 27.8) | .030 c | .933 |
| HOS-ADL | 87.0 (83.9 to 90.2) | 16.7 (12.4 to 21.0) | 92.6 (89.8 to 95.5) | 19.3 (15.4 to 23.2) | .010 c | .378 |
| HOS-SS | 68.2 (62.3 to 74.2) | 29.5 (22.5 to 36.5) | 77.6 (72.1 to 83.1) | 32.0 (25.6 to 38.4) | .025 c | .603 |
| NAHS | 84.2 (81.1 to 87.4) | 23.9 (19.8 to 28.0) | 89.8 (86.9 to 92.7) | 20.8 (17.0 to 24.5) | .011 c | .273 |
| iHOT-33 | 70.6 (66.0 to 75.1) | 34.9 (29.2 to 40.5) | 79.8 (75.6 to 83.9) | 34.7 (29.5 to 39.8) | .004 c | .960 |
| VAS | 2.4 (1.9 to 2.9) | −3.4 (−4.1 to −2.7) | 1.6 (1.2 to 2.1) | −2.9 (−3.5 to −2.2) | .027 c | .261 |
| 24 Months | ||||||
| mHHS | 83.5 (80.3 to 86.7) | 24.4 (20.5 to 28.3) | 88.0 (85.1 to 91.0) | 23.6 (20.1 to 27.1) | .041 c | .768 |
| HOS-ADL | 87.4 (84.2 to 90.5) | 17.4 (13.1 to 21.7) | 93.0 (90.1 to 95.8) | 19.6 (15.7 to 23.6) | .010 c | .448 |
| HOS-SS | 74.5 (68.6 to 80.5) | 36.1 (29.1 to 43.0) | 80.2 (74.7 to 85.6) | 34.6 (28.2 to 40.9) | .170 | .754 |
| NAHS | 84.4 (81.3 to 87.5) | 24.3 (20.2 to 28.4) | 90.8 (87.9 to 93.7) | 21.6 (17.9 to 25.4) | .003 c | .354 |
| iHOT-33 | 71.6 (67.1 to 76.1) | 36.3 (30.7 to 41.9) | 80.9 (76.7 to 85.0) | 35.9 (30.8 to 41.0) | .003 c | .910 |
| VAS | 2.5 (2.0 to 3.0) | −3.4 (−4.1 to −2.8) | 1.7 (1.3 to 2.1) | −2.9 (−3.5 to −2.3) | .017 c | .268 |
Patient-reported outcome scores and interval improvements are reported as mean (95% CI). HOS-ADL, Hip Outcome Score–Activities of Daily Living; HOS-SS, Hip Outcome Score–Sports Subscale; HS, hip-spine; iHOT-33, International Hip Outcome Tool–33; MC, matched control; mHHS, modified Harris Hip Score; NAHS, Non-Arthritic Hip Score; PROM, patient-reported outcome measure; VAS, visual analog scale. Dashes indicate not applicable.
Mean improvement at each time point reported relative to preoperative, baseline score.
A significant difference between groups.
Table 5.
Weighted Differences in Interval PROM/Pain Score Improvement Between Patients With (Hip-Spine) and Without (Matched Control) Hip-Spine Syndrome From Baseline Through 24-Month Follow-up a
| PROM | Weighted Difference in Mean Improvement (95% CI) b | P |
|---|---|---|
| mHHS | −2.5 (–7.7 to 2.7) | .339 |
| HOS-ADL | −1.5 (–7.3 to 4.3) | .609 |
| HOS-SS | 4.6 (–4.8 to 14.0) | .336 |
| NAHS | 4.0 (–1.6 to 9.6) | .161 |
| iHOT-33 | 2.0 (–5.5 to 9.5) | .601 |
| VAS | 0.0 (–0.9 to 0.9) | .981 |
Weighted differences in improvements are reported as mean (95% CI). HOS-ADL, Hip Outcome Score–Activities of Daily Living; HOS-SS, Hip Outcome Score–Sports Subscale; iHOT-33, International Hip Outcome Tool–33; mHHS, modified Harris Hip Score; NAHS, Non-Arthritic Hip Score; PROM, patient-reported outcome measure; VAS, visual analog scale.
Reference group: matched control cohort.
Rates of Achieving Clinically Meaningful Outcomes
When assessing clinically meaningful outcomes, no significant differences were observed between the HS and MC cohorts in terms of the proportion of patients achieving MCID thresholds for mHHS, HOS-ADL, HOS-SS, or iHOT-33 at 12- or 24-month follow-up. The only exception to this trend was a significantly greater percentage of HS patients achieved MCID compared with the MC cohort on the basis of the NAHS (HS: 83.1% vs MC: 67.1%; P = .030) at 12 months; however, this difference did not persist at 24-month follow-up (HS: 83.3% vs MC: 80.5%; P = .664) (Table 6). For PASS analysis, at 12-month follow-up, significantly fewer patients in the HS cohort achieved the clinical threshold relative to MC patients across all collected PROMs. At 24-month follow-up, this pattern largely continued for all PROMs with the exception of the HOS-SS (59.1% vs 68.8%; P = .225) (Table 6).37,45
Table 6.
Frequency of HS and MC Patients Achieving MCID and PASS Thresholds a
| MCID | PASS | |||||||
|---|---|---|---|---|---|---|---|---|
| Threshold | HS Cohort % Achieved | MC Cohort % Achieved | P | Threshold | HS Cohort % Achieved | MC Cohort % Achieved | P | |
| 12 Months | n = 65 | n = 76 | n = 65 | n = 76 | ||||
| mHHS | Δ >6.9 | 87.7 | 81.3 | .303 | >84.8 | 44.6 | 61.3 | .048 b |
| HOS-ADL | Δ >8.8 | 67.7 | 68.4 | .926 | >89.7 | 49.2 | 81.6 | <.001 b |
| HOS-SS | Δ >13.9 | 78.5 | 73.3 | .480 | >72.2 | 47.7 | 68.0 | .015 b |
| NAHS | Δ >9.1 | 83.1 | 67.1 | .030 b | >81.9 | 64.6 | 86.8 | .002 b |
| iHOT-33 | Δ >15.1 | 75.0 | 75.0 | .999 | >69.1 | 54.7 | 80.3 | .001 b |
| 24 Months | n = 66 | n = 77 | n = 66 | n = 77 | ||||
| mHHS | Δ >9.2 | 84.9 | 83.1 | .779 | >83.3 | 59.1 | 75.3 | .038 b |
| HOS-ADL | Δ >9.7 | 62.1 | 68.8 | .399 | >88.2 | 65.2 | 81.8 | .023 b |
| HOS-SS | Δ >14.3 | 78.8 | 77.9 | .900 | >76.4 | 59.1 | 68.8 | .225 |
| NAHS | Δ >8.3 | 83.3 | 80.5 | .664 | >85.6 | 60.6 | 80.5 | .009 b |
| iHOT-33 | Δ >13.9 | 80.3 | 84.4 | .519 | >72.2 | 54.6 | 72.7 | .024 b |
HOS-ADL, Hip Outcome Score–Activities of Daily Living; HOS-SS, Hip Outcome Score–Sports Subscale; HS, hip-spine; iHOT-33, International Hip Outcome Tool–33; MC, matched control; MCID, minimal clinically important difference; mHHS, modified Harris Hip Score; NAHS, Non-Arthritic Hip Score; PASS, Patient Acceptable Symptom State.
A significant difference between groups.
Subgroup Analysis: Minimum 3-Year Follow-up
A total of 89 patients with a minimum 3-year follow-up were included in a subgroup analysis (HSsub, n = 40; MCsub, n = 49) (see Appendix Figure A1, available in the online version of this article). The mean (95% CI) length of follow-up was 48.1 (44.6-51.7) and 50.2 (47.1-53.3) months for the HSsub and MCsub cohorts, respectively, and 49.3 (46.9, 51.6) months for the entire subgroup. No significant differences were identified between cohorts in patient demographic/preoperative characteristics, intraoperative findings, or arthroscopic procedures performed (Appendix Tables A1 and A2, available online).
Discrepancies in mean PROM and VAS pain scores between cohorts nominally diminished over longer follow-up. Specifically, by 3-year follow-up (HSsub, n = 40; MCsub, n = 49; 100% of the subgroup), no significant differences were observed in mean (95% CI) HOS-ADL (88.3 [83.8-92.8] vs 91.0 [86.9-95.2]; Pmean = .384), HOS-SS (75.3 [66.3-84.3] vs 78.1 [69.8-86.4]; Pmean = .651), or NAHS (85.6 [81.2-90.1] vs 90.2 [86.0-94.3]; Pmean = .147), but HSsub patients maintained significantly worse scores in mHHS (82.1 [77.6-86.6] vs 90.2 [86.0-94.5]; Pmean = .010), iHOT-33 (71.4 [4.4-78.3] vs 81.3 [75.0-87.6]; Pmean = .039), and VAS pain (2.7 [1.9-3.4] vs 1.3 [0.6-2.0]; Pmean = .010) (Appendix Table A3, available online). However, exploratory analyses (HSsub, n = 21; MCsub, n = 31; 58.4% of the subgroup) found no significant differences between cohorts in any collected outcomes at 5-year follow-up (Appendix Table A3, available online). Linear mixed-effects models incorporating time as a categorical variable revealed no significant differences between HSsub and MCsub patients in magnitudes of improvement for all functional and pain metrics when tracked from baseline to 5 years (Appendix Table A4, available online). Finally, regarding MCID and PASS thresholds at 5 years, no significant differences were noted for any PROM within the limited available sample (Appendix Table A5, available online).37,45
Subsequent Surgeries and Patient Satisfaction
Patient satisfaction, rates of revision hip arthroscopy, and rates of conversion to THA were tracked for all patients (N = 157) through the maximum available follow-up (HS: 38.2 months vs MC: 39.2 months). When evaluating patient satisfaction via a binary metric (ie, yes/no—are you satisfied with the treatment you received?), no significant difference was found between cohorts regarding the percentage of satisfied patients (HS: 70.0% vs MC: 63.2%) between cohorts (P = .372). With regard to rates of revision hip arthroscopy, no significant difference (P = .999) was found, as no patients (0%) in the HS cohort underwent a revision procedure compared with 1 (1.1%) in the MC cohort. In addition, no significant difference (P = .325) was found with respect to conversion to THA, with 3 (4.3%) HS patients opting for arthroplasty at a mean (95% CI) of 3.06 (1.7-4.4) years versus 1 (1.1%) MC patient at 4.25 years after primary hip arthroscopy. Finally, overall reoperation rates of 4.3% (n = 3) and 2.3% (n = 2) for the HS and MC cohorts, respectively, were not significantly different (P = .657).
Discussion
For patients with FAI and concomitant lumbosacral symptoms, current literature largely indicates a need for additional studies to better guide preoperative education and the shared decision-making process. 50 The results of this study demonstrated that both HS and MC patients achieved significant functional improvement in all PROMs through their 24-month follow-up, with comparable rates of revision hip arthroscopy, conversion to THA, and self-reported satisfaction at maximum available follow-up. Although patients with lumbosacral pathology (HS cohort) were generally found to have nominally worse pre- and postoperative PROMs compared with MC patients, both cohorts were found to exhibit similar magnitudes of improvement over time. In addition, the subgroup analysis of the 40 HSsub and 49 MCsub patients tracked beyond 24 months suggests that patients with concurrent lumbosacral pathologies can continue to experience improvement at 3- and 5-year follow-ups. These findings suggest that mid- to long-term follow-up may be necessary to accurately define clinically meaningful outcomes in the setting of arthroscopic hip preservation surgery.
Furthermore, defining clinically meaningful outcomes has gained prominence in orthopaedic literature and represents a spectrum of improvement perceived by patients. Numerous studies have demonstrated that while MCID represents the lower boundary of improvement relative to PASS or “substantial clinical benefit” thresholds, its use can be appropriately applied to compare outcomes between groups to define the clinical change that is meaningful and detectable to patients.4,21,37 As such, while patients with HS syndrome achieved PASS thresholds at a significantly lower rate at 24-month follow-up, our limited subgroup analysis of midterm data trended toward statistically similar proportions at 5-year follow-up. In addition, MCID analysis revealed that the HS and MC cohorts achieved threshold values at nearly uniform rates across 12- and 24-month follow-ups, and the limited midterm (5-year) follow-up suggested a similar trend. These findings offer insight into the recovery timeline of patients with variations of HS syndrome and indicate that clinically meaningful outcomes can be achieved despite preexisting lumbosacral spine pathology.
Given the variable presentation of symptoms in patients with hip and spine disorders, the preoperative workup is critical to identify appropriate surgical candidates. In addition to a thorough physical examination, including provocative maneuvers specific to both sites, an intra-articular hip joint injection of local anesthetic with a low-dose corticosteroid is a powerful diagnostic tool that should be routinely employed in patients with HS syndrome. 27 For patients with limited clinical benefit after the injection or for those with gross complaints of radicular/sciatic symptoms, the senior author recommends a multidisciplinary approach with an early referral to a dedicated spine surgeon and/or physiatrist— prior to any surgical intervention of the hip. Furthermore, for patients with positive responses to the intra-articular injection, the temporary pain relief may enhance their ability to participate in preoperative physical therapy and facilitate muscle strengthening of the core/hemipelvis to optimize their recovery after hip arthroscopy. 35
To date, the lack of homogeneity among available literature has provided conflicting evidence regarding the influence of lumbosacral disease on hip arthroscopy outcomes. For example, Chandrasekaran et al 9 reported that previous lumbar spine fusion and/or decompression (n = 57) did not adversely affect 24-month outcomes in a 1:1 matched-pair controlled study, but a variety of intra-articular diagnoses and concomitant procedures performed limited generalizability to any specific patient population. Conversely, in a nested case-control analysis, Beck et al 4 found that despite displaying similar PROM scores at baseline, a cohort of patients with lumbosacral spine pathology (n = 83) displayed significantly lower PROM scores at 24-month follow-up relative to the matched-control cohort (n = 166). However, the clinical application of these findings is clouded by one-fourth (25.3%) of the comparison cohort receiving previous surgical treatment for lumbosacral pain, which has been reported to be an independent predictor of poor outcomes after hip arthroscopy. 4 Specifically, Feingold et al 14 provided preliminary evidence (n = 20; 14 fusion and 6 laminectomies/discectomies) that previous lumbosacral interventions prognosticated worse outcomes for subsequent hip preservation surgery, with subanalyses clearly delineating suboptimal outcomes in patients with previous fusion. These findings are further corroborated in spine-specific literature that has associated lumbar fusion with loss of lumbar mobility, risk of adjacent segment disease, and reduced spinopelvic capacity to compensate during postural changes.8,19,51 Overall, variations in patient characteristics, short-term follow-up, and small sample sizes have limited the generalizability of findings available in the current literature.
This present study leverages a robust collection of prospective data to complement the limited data that exist comparing outcomes of arthroscopic labral repair in the setting of FAI and concurrent lumbosacral symptomatology. A major strength of this analysis includes the strict application of eligibility criteria to appropriately identify a homogeneous cohort of patients with objective evidence (eg, imaging with radiologic reports) of lumbosacral pathologies without any history of spine surgery. In addition, all patients had a diagnosis of a symptomatic labral tear secondary to FAI. Both cohorts received similar and comprehensive preoperative workups; had ≥3 months of unsuccessful nonoperative treatment, including physical therapy; underwent hip arthroscopy by a single, high-volume, fellowship-trained orthopaedic surgeon using a uniform surgical approach; and progressed through the same protocol postoperatively. Furthermore, outcomes were tracked across multiple validated PROMs with additional consideration for the rates of achieving MCID and PASS thresholds. 39 Finally, CEM was used to minimize confounding variables, reduce the risk of selection bias, and maximize the number of patients within each cohort.
Limitations
Despite the strengths of this current study, there are certain limitations to address. First, the findings reported were those of the senior author who had already performed over 1000 hip arthroscopies prior to May 2014. While this mitigated any risk of expert bias within the study cohorts, these outcomes may not be generalizable to all surgeons who use different techniques, especially in regard to capsular management, methods of labral reconstruction, and treatment of chondrolabral/chondral injury.10,28,32,36,38 Second, while we obtained a host of hip-specific PROMs, quantitative evaluation of lower back symptoms and subsequent improvement using validated metrics postoperatively remains an important area of future investigation. In addition, while outside the scope of this study, tracking dynamic changes in spinopelvic parameters during the postoperative period and correlation with patient-specific factors may better inform outcomes in the setting of HS syndrome. Third, commensurate with available studies, further subanalyses could not be performed to assess the effect of each specific lumbosacral pathology without being grossly underpowered. 4 Fourth, MCID and PASS analyses were limited to patients with discrete follow-up at the corresponding threshold time points. Similarly, commensurate with other retrospective outcomes studies, 62 patients could not be analyzed due to missing/incomplete PROM scores, which may have introduced the possibility of selection bias. Last, we report a limited number of 3- and 5-year (mid-term) outcomes to showcase that longer follow-up may be necessary to accurately gauge the clinical benefit achieved after hip preservation surgery and provide a preliminary benchmark for future research assessing mid-term outcomes.
Conclusion
After hip arthroscopy to address labral tears in the setting of FAI, patients with symptomatic lumbosacral pathologies and no history of spine surgery were found to exhibit inferior pre- and postoperative PROMs but achieved statistically similar clinical benefit and rates of PROM improvement through 24-month follow-up compared with matched controls with isolated hip disease. These findings aid in providing a realistic recovery timeline and evidence that coexisting hip and spine disorders are not a contraindication for arthroscopic hip preservation surgery.
Supplemental Material
Supplemental material, sj-pdf-1-ajs-10.1177_03635465231197374 for Outcomes of Hip Arthroscopy in the Setting of Concomitant Symptomatic Lumbosacral Spine Pathology by Kaveh A. Torabian, Nathan J. Cherian, Michael C. Dean, Christopher T. Eberlin, Michael P. Kucharik, Kieran S. Dowley, Zachary L. LaPorte and Scott D. Martin in The American Journal of Sports Medicine
Acknowledgments
The authors thank the Conine Family Foundation for Joint Preservation for its support and Mark Cote, DPT, MS, for assistance with statistical analysis.
Footnotes
Submitted February 7, 2023; accepted July 20, 2023.
One or more of the authors has declared the following potential conflict of interest or source of funding: S.D.M. has received support for education from Kairos Surgical and a gift from Allergan. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Presented as a poster at the annual meeting of the AOSSM, Washington, DC, July 2023.
ORCID iDs: Kaveh A. Torabian  https://orcid.org/0000-0003-4265-7345
https://orcid.org/0000-0003-4265-7345
Nathan J. Cherian  https://orcid.org/0000-0002-1524-3908
https://orcid.org/0000-0002-1524-3908
Michael C. Dean  https://orcid.org/0000-0001-5996-6393
https://orcid.org/0000-0001-5996-6393
Kieran S. Dowley  https://orcid.org/0000-0002-1758-9929
https://orcid.org/0000-0002-1758-9929
References
- 1. Akpinar B, Vasavada K, Rynecki ND, Owusu-Sarpong S, Youm T. Hip spine syndrome negatively impacts arthroscopic outcomes in the management of femoroacetabular impingement syndrome: a systematic review. Arthroscopy. 2023;39(6):1552-1564. [DOI] [PubMed] [Google Scholar]
- 2. Alpaugh K, Shin SR, Martin SD. Intra-articular fluid distension for initial portal placement during hip arthroscopy: the “femoral head drop” technique. Arthrosc Techn. 2015;4(1):e23-e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1-48. [Google Scholar]
- 4. Beck EC, Nwachukwu BU, Chapman R, Gowd AK, Waterman BR, Nho SJ. The influence of lumbosacral spine pathology on minimum 2-year outcome after hip arthroscopy: a nested case-control analysis. Am J Sports Med. 2020;48(2):403-408. [DOI] [PubMed] [Google Scholar]
- 5. Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87(7):1012-1018. [DOI] [PubMed] [Google Scholar]
- 6. Becker LC, Carter-Kelley S, Ellis T, Cenkus K, Di Stasi SL. Pre-operative low back pain negatively affects self-reported function in individuals undergoing hip arthroscopy. Int J Sports Phys Ther. 2015;10(7):992-997. [PMC free article] [PubMed] [Google Scholar]
- 7. Brown-Taylor L, Bordner H, Glaws K, Vasileff WK, Walrod B, Di Stasi S. Prevalence of low back pain and related disability in patients with femoroacetabular impingement syndrome. PM R. 2022;14(1):8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bydon M, Macki M, Kerezoudis P, et al. The incidence of adjacent segment disease after lumbar discectomy: a study of 751 patients. J Clin Neurosci. 2017;35:42-46. [DOI] [PubMed] [Google Scholar]
- 9. Chandrasekaran S, Darwish N, Darwish AH, Suarez-Ahedo C, Lodhia P, Domb BG. Outcomes of hip arthroscopy in patients with previous lumbar spine surgery: a matched-pair controlled comparative study with minimum two-year follow-up. Arthroscopy. 2019;35(2):443-450. [DOI] [PubMed] [Google Scholar]
- 10. Eberlin CT, Kucharik MP, Abraham PF, et al. Puncture capsulotomy technique for hip arthroscopy: midterm functional outcomes. Orthop J Sports Med. 2023;11(1):232596712211440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Esposito CI, Carroll KM, Sculco PK, Padgett DE, Jerabek SA, Mayman DJ. Total hip arthroplasty patients with fixed spinopelvic alignment are at higher risk of hip dislocation. J Arthroplasty. 2018;33(5):1449-1454. [DOI] [PubMed] [Google Scholar]
- 12. Esposito CI, Miller TT, Kim HJ, et al. Does degenerative lumbar spine disease influence femoroacetabular flexion in patients undergoing total hip arthroplasty? Clin Orthop Relat Res. 2016;474(8):1788-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fader RR, Tao MA, Gaudiani MA, et al. The role of lumbar lordosis and pelvic sagittal balance in femoroacetabular impingement. Bone Joint J. 2018;100(10):1275-1279. [DOI] [PubMed] [Google Scholar]
- 14. Feingold JD, Heaps B, Turcan S, Swartwout E, Ranawat A. A history of spine surgery predicts a poor outcome after hip arthroscopy. J Hip Preserv Surg. 2019;6(3):227-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Feingold JD, Srikumar S, Vaswani R, White AE, Swartwout EL, Ranawat AS. The outcome of hip arthroscopy in the setting of lumbar spine disease is beneficial, yet limited: a systematic review of existing evidence. Arthroscopy. 2023;39(6):1568-1583. [DOI] [PubMed] [Google Scholar]
- 16. Guy D, Karp I, Wilk P, Chin J, Rodrigues G. Propensity score matching versus coarsened exact matching in observational comparative effectiveness research. J Comp Eff Res. 2021;10(11):939-951. [DOI] [PubMed] [Google Scholar]
- 17. Haldane CE, Ekhtiari S, de SA D, Simunovic N, Ayeni OR. Preoperative physical examination and imaging of femoroacetabular impingement prior to hip arthroscopy—a systematic review. J Hip Preserv Surg. 2017;4(3):201-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Haskel JD, Baron SL, Zusmanovich M, Youm T. Does concomitant lumbar spine disease adversely affect the outcomes of patients undergoing hip arthroscopy? Am J Sports Med. 2020;48(9):2178-2184. [DOI] [PubMed] [Google Scholar]
- 19. Helgeson MD, Bevevino AJ, Hilibrand AS. Update on the evidence for adjacent segment degeneration and disease. Spine J. 2013;13(3):342-351. [DOI] [PubMed] [Google Scholar]
- 20. Iacus SM, King G, Porro G. Causal inference without balance checking: coarsened exact matching. Polit Anal. 2012;20(1):1-24. [Google Scholar]
- 21. Katz NP, Paillard FC, Ekman E. Determining the clinical importance of treatment benefits for interventions for painful orthopedic conditions. J Orthop Surg Res. 2015;10(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kucharik MP, Abraham PF, Nazal MR, Varady NH, Meek WM, Martin SD. Minimum 2-year functional outcomes of patients undergoing capsular autograft hip labral reconstruction. Am J Sports Med. 2021;49(10):2659-2667. [DOI] [PubMed] [Google Scholar]
- 23. Lazennec JY, Brusson A, Rousseau MA. Hip-spine relations and sagittal balance clinical consequences. Eur Spine J. 2011;20(suppl 5):686-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee H, Lim W, Lee S, Jo S, Jo S. Impact of capsulotomy on hip biomechanics during arthroscopy. Medicina. 2022;58(10):1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee MS, Mahatme RJ, Simington J, et al. Over 50% of studies report low back pain is associated with worse outcomes after hip arthroscopy when compared to a control group: a systematic review. Published online May 18, 2023. Arthroscopy. doi: 10.1016/j.arthro.2023.05.004 [DOI] [PubMed] [Google Scholar]
- 26. Luthringer TA, Vigdorchik JM. A preoperative workup of a “hip-spine” total hip arthroplasty patient: a simplified approach to a complex problem. J Arthroplasty. 2019;34(7):S57-S70. [DOI] [PubMed] [Google Scholar]
- 27. Maldonado DR, Mu BH, Ornelas J, et al. Hip-spine syndrome: the diagnostic utility of guided intra-articular hip injections. Orthopedics. 2020;43(2):e65-e71. [DOI] [PubMed] [Google Scholar]
- 28. Martin SD, Eberlin CT, Kucharik MP, Cherian NJ. Harvest and application of bone marrow aspirate concentrate to address acetabular chondral damage during hip arthroscopy. JBJS Essent Surg Tech. 2023;13(2):e22.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martin SD, Kucharik MP, Abraham PF, Nazal MR, Meek WM, Varady NH. Functional outcomes of arthroscopic acetabular labral repair with and without bone marrow aspirate concentrate. J Bone Joint Surg Am. 2022;104(1):4-14. [DOI] [PubMed] [Google Scholar]
- 30. Mayer SW, Fauser TR, Marx RG, et al. Reliability of the classification of cartilage and labral injuries during hip arthroscopy. J Hip Preserv Surg. 2020;7(3):448-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McCormick F, Alpaugh K, Nwachukwu BU, Xu S, Martin SD. Effect of radiofrequency use on hip arthroscopy irrigation fluid temperature. Arthroscopy. 2013;29(2):336-342. [DOI] [PubMed] [Google Scholar]
- 32. Mehta N, Chamberlin P, Marx RG, et al. Defining the learning curve for hip arthroscopy: a threshold analysis of the volume-outcomes relationship. Am J Sports Med. 2018;46(6):1284-1293. [DOI] [PubMed] [Google Scholar]
- 33. Meyer DC, Beck M, Ellis T, Ganz R, Leunig M. Comparison of six radiographic projections to assess femoral head/neck asphericity. Clin Orthop Relat Res. 2006;445:181-185. [DOI] [PubMed] [Google Scholar]
- 34. Montgomery SR, Ngo SS, Hobson T, et al. Trends and demographics in hip arthroscopy in the United States. Arthroscopy. 2013;29(4):661-665. [DOI] [PubMed] [Google Scholar]
- 35. Naessig S, Kucharik M, Meek W, Eberlin C, Martin S. Prehabilitation and rehabilitation program for patients undergoing arthroscopic acetabular labral repair: a comprehensive 5-phase patient-guided program. Orthop J Sports Med. 2022;10(2):232596712110710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nwachukwu BU, Alpaugh K, McCormick F, Martin SD. All-arthroscopic reconstruction of the acetabular labrum by capsular augmentation. Arthrosc Techn. 2015;4(2):e127-e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nwachukwu BU, Beck EC, Kunze KN, Chahla J, Rasio J, Nho SJ. Defining the clinically meaningful outcomes for arthroscopic treatment of femoroacetabular impingement syndrome at minimum 5-year follow-up. Am J Sports Med. 2020;48(4):901-907. [DOI] [PubMed] [Google Scholar]
- 38. Nwachukwu BU, McCormick F, Martin SD. Arthroscopic technique for chondrolabral capsular preservation during labral repair and acetabular osteoplasty. Arthrosc Techn. 2013;2(3):e213-e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nwachukwu BU, Rasio J, Beck EC, et al. Patient-reported outcomes measurement information system physical function has a lower effect size and is less responsive than legacy hip specific patient reported outcome measures following arthroscopic hip surgery. Arthroscopy. 2020;36(12):2992-2997. [DOI] [PubMed] [Google Scholar]
- 40. Offierski CM, MacNab I. Hip-spine syndrome. Spine. 1983;8(3):316-321. [DOI] [PubMed] [Google Scholar]
- 41. Quinlan NJ, Alpaugh K, Upadhyaya S, Conaway WK, Martin SD. Improvement in functional outcome scores despite persistent pain with 1 year of nonsurgical management for acetabular labral tears with or without femoroacetabular impingement. Am J Sports Med. 2019;47(3):536-542. [DOI] [PubMed] [Google Scholar]
- 42. Raudenbush SW. Comparing personal trajectories and drawing causal inferences from longitudinal data. Ann Rev Psychol. 2001;52(1):501-525. [DOI] [PubMed] [Google Scholar]
- 43. Redmond JM, Gupta A, Hammarstedt JE, Stake CE, Domb BG. The hip-spine syndrome: how does back pain impact the indications and outcomes of hip arthroscopy? Arthroscopy. 2014;30(7):872-881. [DOI] [PubMed] [Google Scholar]
- 44. Ripollone JE, Huybrechts KF, Rothman KJ, Ferguson RE, Franklin JM. Evaluating the utility of coarsened exact matching for pharmacoepidemiology using real and simulated claims data. Am J Epidemiol. 2020;189(6):613-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rosinsky PJ, Kyin C, Maldonado DR, et al. Determining clinically meaningful thresholds for the nonarthritic hip score in patients undergoing arthroscopy for femoroacetabular impingement syndrome. Arthroscopy. 2021;37(10):3113-3121. [DOI] [PubMed] [Google Scholar]
- 46. Savage TN, Saxby DJ, Lloyd DG, et al. Hip contact force magnitude and regional loading patterns are altered in those with femoroacetabular impingement syndrome. Med Sci Sports Exerc. 2022;54(11):1831-1841. [DOI] [PubMed] [Google Scholar]
- 47. Skelley NW, Conaway WK, Martin SD. “In-round” labral repair after acetabular recession using intermittent traction. Arthrosc Techn. 2017;6(5):e1807-e1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Snijders TAB, Bosker RJ. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. Sage; 2011. [Google Scholar]
- 49. Syed HM, Martin SD. Arthroscopic acetabular recession with chondrolabral preservation. Am J Orthop (Belle Mead NJ). 2013;42(4):181-184. [PubMed] [Google Scholar]
- 50. Vaswani R, White AE, Feingold J, Ranawat AS. Hip-spine syndrome in the nonarthritic patient. Arthroscopy. 2022;38(10):2930-2938. [DOI] [PubMed] [Google Scholar]
- 51. Wang S, Zhou Q, Xu L, et al. Impact of lumbar fusion on sitting spinopelvic balance: multisegmental versus monosegmental. Clin Neurol Neurosurg. 2021;209:106905. [DOI] [PubMed] [Google Scholar]
- 52. Yacovelli S, Sutton R, Vahedi H, Sherman M, Parvizi J. High risk of conversion to THA after femoroacetabular osteoplasty for femoroacetabular impingement in patients older than 40 years. Clin Orthop Relat Res. 2021;479(5):1112-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ajs-10.1177_03635465231197374 for Outcomes of Hip Arthroscopy in the Setting of Concomitant Symptomatic Lumbosacral Spine Pathology by Kaveh A. Torabian, Nathan J. Cherian, Michael C. Dean, Christopher T. Eberlin, Michael P. Kucharik, Kieran S. Dowley, Zachary L. LaPorte and Scott D. Martin in The American Journal of Sports Medicine