Fig. 2 |. Subcellular structures and organelles regulating progenitor polarity.
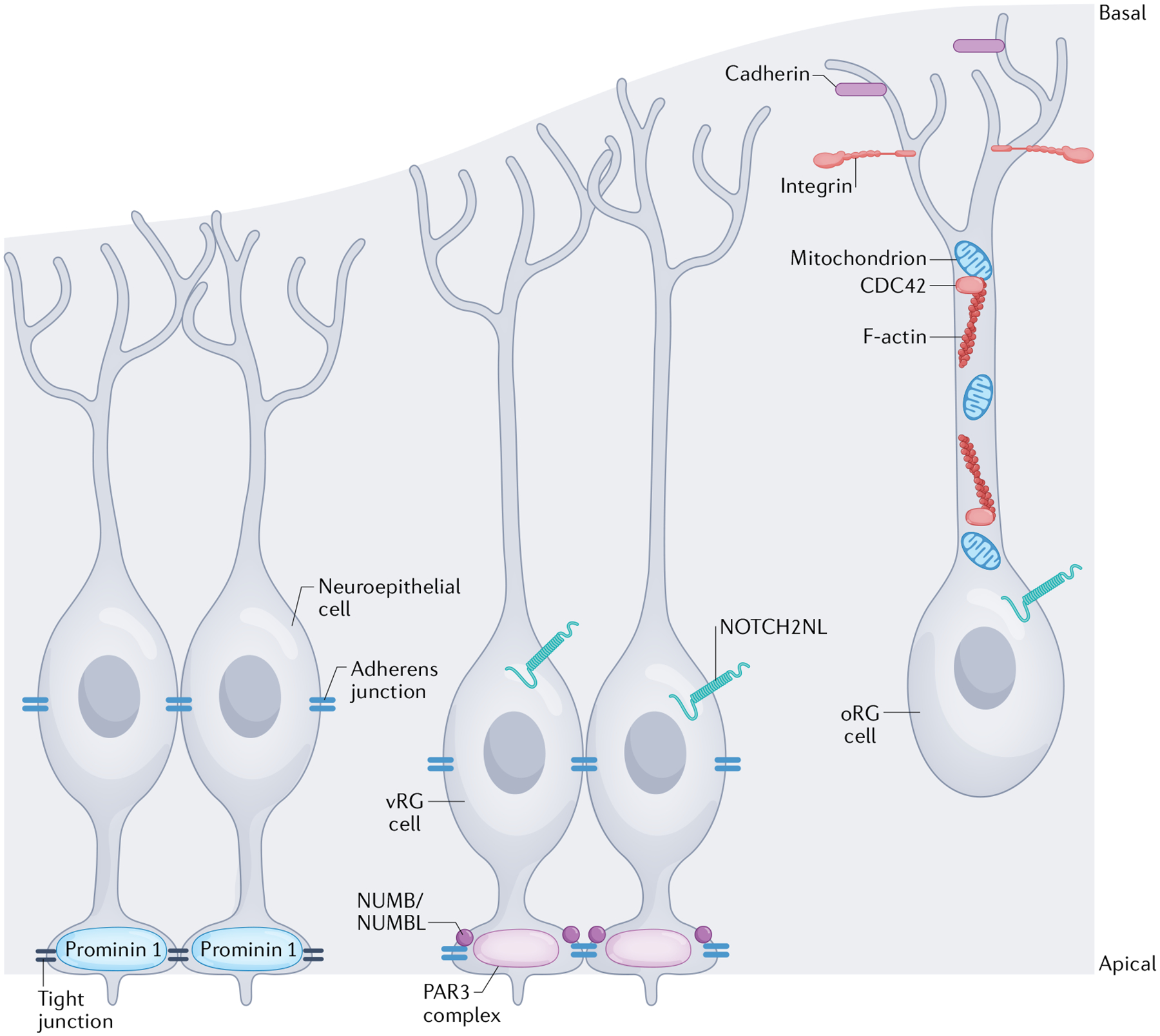
The specific morphology and polarity of different cortical progenitor subtypes are regulated by distinct molecular mechanisms. Although the dynamic regulation of human progenitor polarity is being revealed, most mechanistic studies have been performed in rodent or other model species. This figure represents a cumulative understanding of polarity from animal and human studies. The apical membranes of neuroepithelial cells form the ventricular surface of the cortex. Neuroepithelial cells express the transmembrane glycoprotein prominin 1 at the apical membrane168 and tight junctions form between neuroepithelial cells to establish the ventricular surface and establish their polarity. In a more basal location within the cells, along the cell body, adherens junctions are also formed between neuroepithelial cells60,61,63. Ventricular radial glial (vRG) cells have a similar apical–basal polarity, but their apical anchoring to the ventricular surface is regulated by a different set of proteins, including those that comprise the PAR3 complex, and the vRG cells are predominantly connected to one another by adherens junctions75. The proteins NUMB and/or NUMBL help maintain these adherens junctions at the apical surface74. Junctional complexes regulate receipt of NOTCH signals, which are involved in mediating proliferation and polarity. The human-specific receptor, NOTCH2NL, impacts radial glial cell proliferation76,78. Outer radial glial (oRG) cells retain a basal polarity, but without an apical attachment. Their connection to the basal lamina is regulated by cadherins and integrins65,67 in the oRG cell endfeet and the integrity of their basal processes is maintained by appropriate F-actin activity (regulated by the GTPase CDC42) and mitochondrial function52,82.
