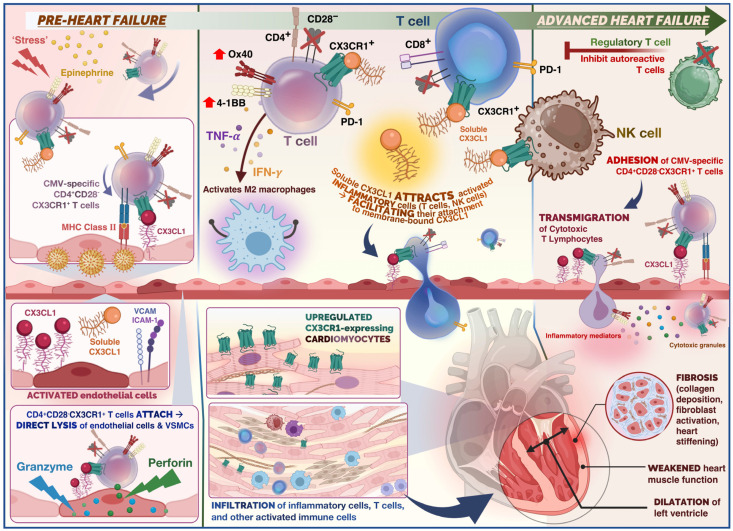
Figure 1.
Summary of the role of immune cells in the cardiovascular disease continuum from the pre-heart failure to advanced heart failure stage in dilated cardiomyopathy (DCM). Physiological stress and release of epinephrine in the acute phase (pre-heart failure), triggers release of CX3CR1+ T cells. In the case of stress-induced viral reactivation, rapid mobilisation for CMV-specific T cells occurs. CX3CR1+ CMV-specific CD4+ T cells would adhere to endothelium, and undergo antigen recognition, leading to activation of cytotoxic effector cells. Direct lysis of endothelial cells and vascular smooth muscle cells (VSMCs) occurs (mediated by granzymes and perforin). Release of cytokines such as TNF-α and IFN-γ as well as chemokines from CMV-specific CD4+ T cells potentially attracts further immune cells such as polarised macrophages and NK cells through endothelial induction of fractalkine, which may enhance endothelial injury. The CX3CR1– regulatory T cells (Tregs) are capable of inhibiting autoreactive T cells. Thus, administration of Tregs serves as a potential therapeutic target to promote immune tolerance. Soluble fractalkine binds to its chemokine receptor on cytotoxic T cells and NK cells which facilitates adhesion and transmigration across the endothelium. Infiltrating T cells and inflammatory cells release inflammatory mediators and cytotoxic granules which promote remodelling, collagen deposition, and fibrosis. This ultimately results in weakening of the ventricular heart muscle and ventricular dilatation.