Figure 7. G007-LK synergizes with cabozantinib to inhibit c-Met/sgAxin1 tumor development.
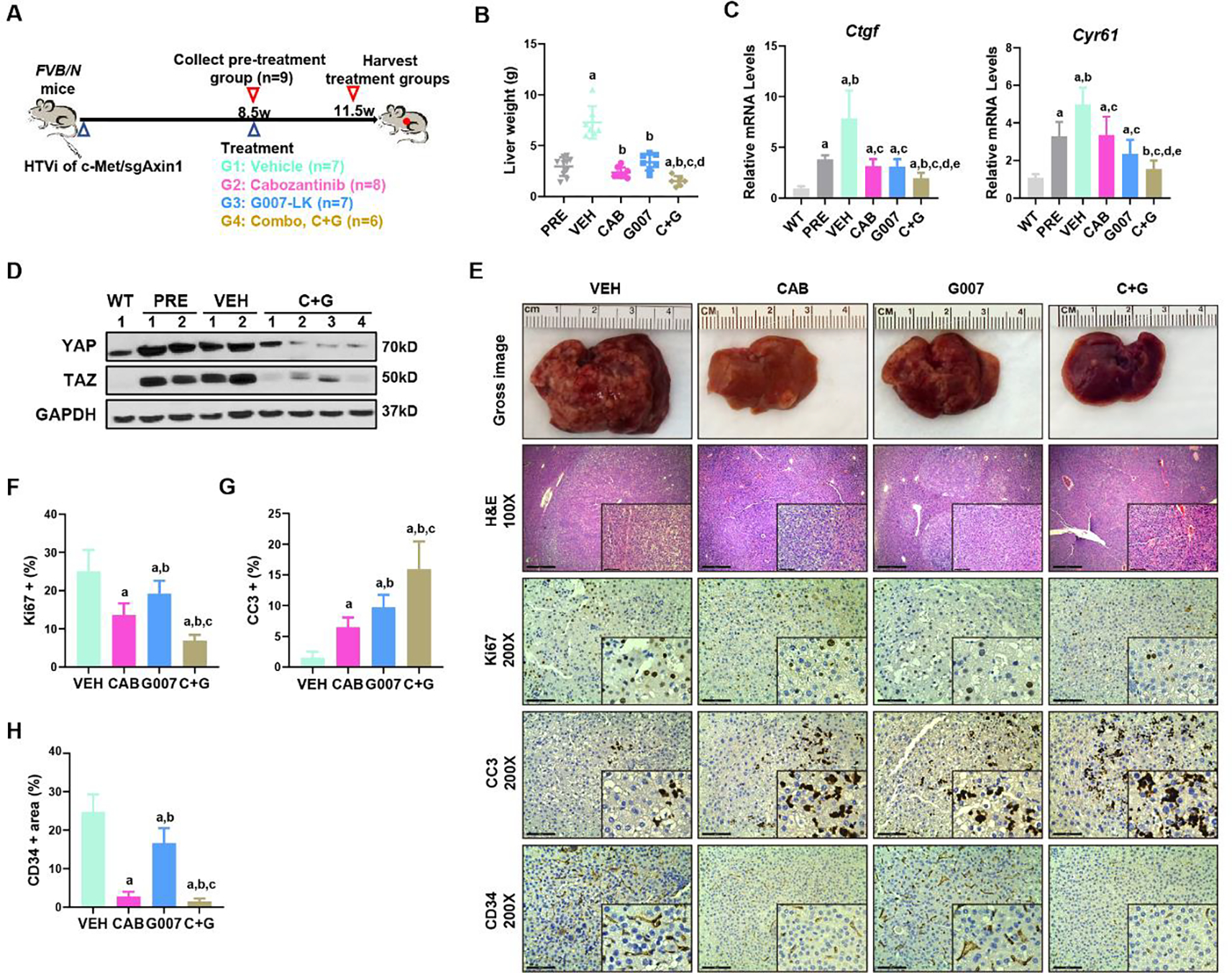
(A) Study design. FVB/N mice were hydrodynamically injected (HTVi) with c-Met/sgAxin1/SB constructs. At 8.5 weeks after injection, one group of mice (n=9) were sacrificed, and liver tissues were harvested as pre-treatment. Other mice were randomly assigned into the vehicle (VEH, n=7), Cabozantinib (CAB, n=8), G007-LK (G007, n=7), or Cabozantinib and G007-LK combinational (C+G, n=6) treated groups. Mice were treated for three weeks and subsequently were sacrificed. (B) Comparisons of the liver weight in the five groups. Mean ± SD; One-way ANOVA test. p<0.05, (a) vs PRE; (b) vs.VEH; (c) vs. CAB; (d) vs. G007. (C) qPCR results of Ctgf and Cyr61 in the normal liver (WT), pre-treatment (PRE), vehicle (VEH), and drug-treated liver tumors. Mean ± SD; One-way ANOVA test. p<0.05, (a) vs WT; (b) vs.PRE; (c) vs. VEH; (d) vs. CAB; (e) vs. G007. (D) Expression of YAP and TAZ determined by Western blot analysis. GAPDH was used as a loading control. (E) Representative images of macroscopic pictures, H&E, Ki-67, Cleaved caspase-3 (CC3), and CD34 staining of the tumors in the four groups. Scale bars: 200μm for 100X, 100μm for 200X. (F-H) Quantifications of Ki67 (F), CC3 (G), and CD34 (H) positive percentage in the four groups. Mean ± SD; One-way ANOVA test. p<0.05, (a) vs VEH; (b) vs. CAB; (c) vs. G007.
