Table 1.
Baseline Demographic and Disease Characteristics of the Modified Intention-to-Treat Population
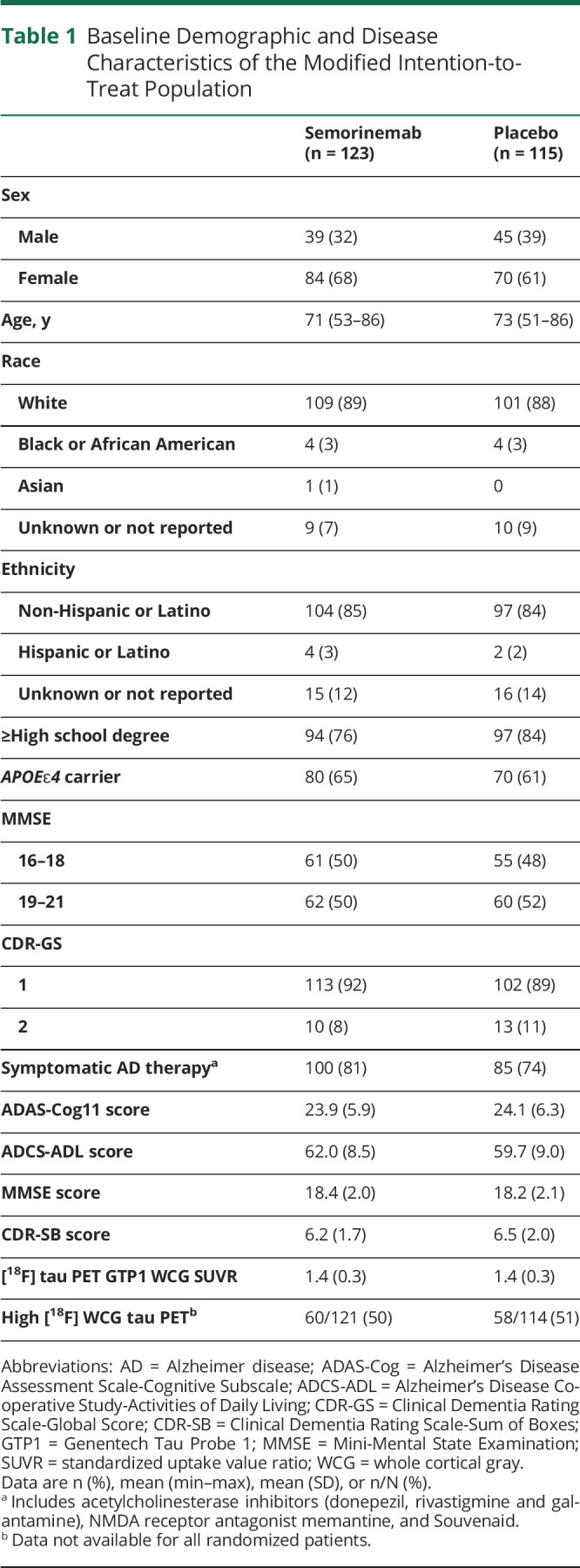
| Semorinemab (n = 123) | Placebo (n = 115) | |
| Sex | ||
| Male | 39 (32) | 45 (39) |
| Female | 84 (68) | 70 (61) |
| Age, y | 71 (53–86) | 73 (51–86) |
| Race | ||
| White | 109 (89) | 101 (88) |
| Black or African American | 4 (3) | 4 (3) |
| Asian | 1 (1) | 0 |
| Unknown or not reported | 9 (7) | 10 (9) |
| Ethnicity | ||
| Non-Hispanic or Latino | 104 (85) | 97 (84) |
| Hispanic or Latino | 4 (3) | 2 (2) |
| Unknown or not reported | 15 (12) | 16 (14) |
| ≥High school degree | 94 (76) | 97 (84) |
| APOEɛ4 carrier | 80 (65) | 70 (61) |
| MMSE | ||
| 16–18 | 61 (50) | 55 (48) |
| 19–21 | 62 (50) | 60 (52) |
| CDR-GS | ||
| 1 | 113 (92) | 102 (89) |
| 2 | 10 (8) | 13 (11) |
| Symptomatic AD therapya | 100 (81) | 85 (74) |
| ADAS-Cog11 score | 23.9 (5.9) | 24.1 (6.3) |
| ADCS-ADL score | 62.0 (8.5) | 59.7 (9.0) |
| MMSE score | 18.4 (2.0) | 18.2 (2.1) |
| CDR-SB score | 6.2 (1.7) | 6.5 (2.0) |
| [18F] tau PET GTP1 WCG SUVR | 1.4 (0.3) | 1.4 (0.3) |
| High [18F] WCG tau PETb | 60/121 (50) | 58/114 (51) |
Abbreviations: AD = Alzheimer disease; ADAS-Cog = Alzheimer's Disease Assessment Scale-Cognitive Subscale; ADCS-ADL = Alzheimer's Disease Cooperative Study-Activities of Daily Living; CDR-GS = Clinical Dementia Rating Scale-Global Score; CDR-SB = Clinical Dementia Rating Scale-Sum of Boxes; GTP1 = Genentech Tau Probe 1; MMSE = Mini-Mental State Examination; SUVR = standardized uptake value ratio; WCG = whole cortical gray.
Data are n (%), mean (min–max), mean (SD), or n/N (%).
Includes acetylcholinesterase inhibitors (donepezil, rivastigmine and galantamine), NMDA receptor antagonist memantine, and Souvenaid.
Data not available for all randomized patients.
