Table 3.
Summary of Treatment-Emergent AEs in Safety Evaluable Population (n = 267) During the Double-Blind Part of the Study (Including the Safety Follow-up Period)
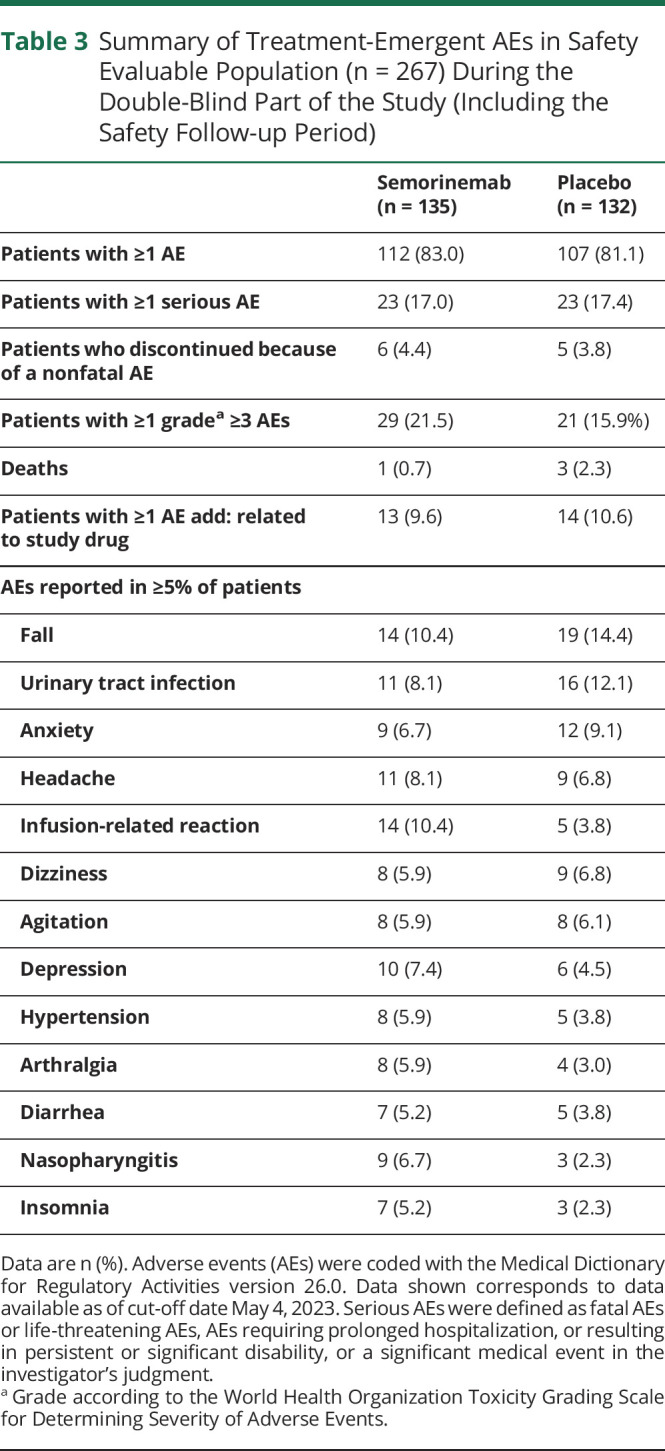
| Semorinemab (n = 135) | Placebo (n = 132) | |
| Patients with ≥1 AE | 112 (83.0) | 107 (81.1) |
| Patients with ≥1 serious AE | 23 (17.0) | 23 (17.4) |
| Patients who discontinued because of a nonfatal AE | 6 (4.4) | 5 (3.8) |
| Patients with ≥1 gradea ≥3 AEs | 29 (21.5) | 21 (15.9%) |
| Deaths | 1 (0.7) | 3 (2.3) |
| Patients with ≥1 AE add: related to study drug | 13 (9.6) | 14 (10.6) |
| AEs reported in ≥5% of patients | ||
| Fall | 14 (10.4) | 19 (14.4) |
| Urinary tract infection | 11 (8.1) | 16 (12.1) |
| Anxiety | 9 (6.7) | 12 (9.1) |
| Headache | 11 (8.1) | 9 (6.8) |
| Infusion-related reaction | 14 (10.4) | 5 (3.8) |
| Dizziness | 8 (5.9) | 9 (6.8) |
| Agitation | 8 (5.9) | 8 (6.1) |
| Depression | 10 (7.4) | 6 (4.5) |
| Hypertension | 8 (5.9) | 5 (3.8) |
| Arthralgia | 8 (5.9) | 4 (3.0) |
| Diarrhea | 7 (5.2) | 5 (3.8) |
| Nasopharyngitis | 9 (6.7) | 3 (2.3) |
| Insomnia | 7 (5.2) | 3 (2.3) |
Data are n (%). Adverse events (AEs) were coded with the Medical Dictionary for Regulatory Activities version 26.0. Data shown corresponds to data available as of cut-off date May 4, 2023. Serious AEs were defined as fatal AEs or life-threatening AEs, AEs requiring prolonged hospitalization, or resulting in persistent or significant disability, or a significant medical event in the investigator's judgment.
Grade according to the World Health Organization Toxicity Grading Scale for Determining Severity of Adverse Events.
