Table 1.
PdI2-catalyzed oxidative aminocarbonylation of 3-(prop-2-yn-1-yl)pentane-2,4-dione 1a under different conditions a.
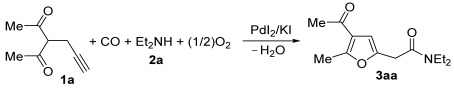
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Solvent | KI (Equiv) |
2a (Equiv) |
Substrate Concn. b | T (°C) | PCO
(atm) |
Pair
(atm) |
Yield of 3aa (%) c |
| 1 | MeCN | 1 | 3 | 0.10 | 100 | 16 | 4 | 61 |
| 2 | dioxane | 1 | 3 | 0.10 | 100 | 16 | 4 | 56 |
| 3 | DMA d | 1 | 3 | 0.10 | 100 | 16 | 4 | 41 |
| 4 | MeCN | 0.5 | 3 | 0.10 | 100 | 16 | 4 | 45 |
| 5 | MeCN | 1 | 2 | 0.10 | 100 | 16 | 4 | 29 |
| 6 | MeCN | 1 | 4 | 0.10 | 100 | 16 | 4 | 68 |
| 7 | MeCN | 1 | 5 | 0.10 | 100 | 16 | 4 | 66 |
| 8 | MeCN | 1 | 3 | 0.20 | 100 | 16 | 4 | 63 |
| 9 | MeCN | 1 | 3 | 0.05 | 100 | 16 | 4 | 51 |
| 10 | MeCN | 1 | 3 | 0.10 | 100 | 32 | 8 | 53 |
| 11 e | MeCN | 1 | 3 | 0.10 | 80 | 16 | 4 | 54 |
| 12 | MeCN | 1 | 4 | 0.20 | 100 | 16 | 4 | 72 |
| 13 f | MeCN | 1 | 4 | 0.20 | 100 | 16 | 4 | 55 |
a Unless otherwise noted, all reactions were carried out in 15 h, using 1 mol% of PdI2. Substrate conversion was quantitative in all cases. b Mmol of starting 1a per mL of solvent. c Isolated yield based on starting 1a. d DMA = N,N-dimethylacetamide. e The GLC analysis showed the presence of trace of substrate. f The reaction was carried out using 0.33 mol% of PdI2.
