Table 2.
Synthesis of 2-(4-acylfuran-2-yl)acetamides 3 by PdI2/KI-catalyzed oxidative aminocarbonylation of 2-propargyl-1,3-dicarbonyl compounds 1 with secondary amines 2 a.

| |||||
|---|---|---|---|---|---|
| Entry | PdI2 (mol%) | 1 | 2 | 3 | Yield of 3 (%) b |
| 1 | 1 |
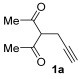
|
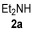
|
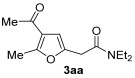
|
72 |
| 2 | 0.33 | 1a | 2a | 3aa | 55 |
| 3 | 1 | 1a |
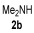
|
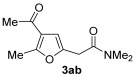
|
67 |
| 4 | 0.33 | 1a | 2b | 3ab | 55 |
| 5 | 1 | 1a |
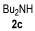
|
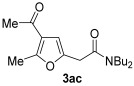
|
75 |
| 6 | 0.33 | 1a | 2c | 3ac | 58 |
| 7 | 1 | 1a |
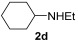
|
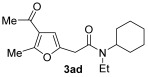
|
74 |
| 8 | 0.33 | 1a | 2d | 3ad | 59 |
| 9 | 1 | 1a |
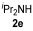
|
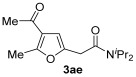
|
54 |
| 10 | 1 | 1a |
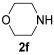
|
|
74 |
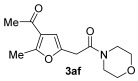
| 11 | 0.33 | 1a | 2f | 3af | 61 |
| 12 | 1 |
|
2a |
|
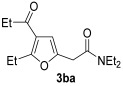
81 |
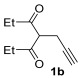
| 13 | 0.33 | 1b | 2a | 3ba | 68 |
| 14 | 1 |
|
2a |
|
67 |
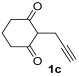
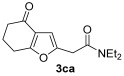
| 15 | 0.33 | 1c | 2a | 3ca | 58 |
| 16 | 1 |
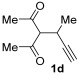
|
2f |
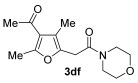
|
66 |
| 17 | 1 |
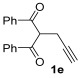
|
2a |
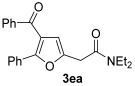
|
68 |
| 18 | 0.33 | 1e | 2a | 3ea | 62 |
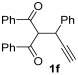
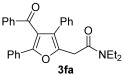
| 19 | 1 |
|
2a |
|
54 |
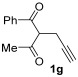
| 20 | 1 |
|
2f |
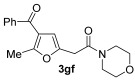
|
48 |
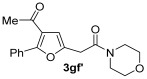
|
25 | ||||
| 21 | 1 |
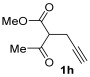
|
2a |
|
70 |
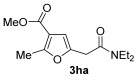
| 22 | 0.33 | 1h | 2a | 3ha | 58 |
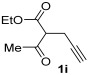
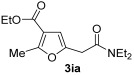
| 23 | 1 |
|
2a |
|
69 |
| 24 | 0.33 | 1i | 2a | 3ia | 58 |
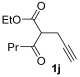
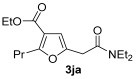
| 25 | 1 |
|
2a |
|
68 |
| 26 | 0.33 | 1j | 2a | 3ja | 63 |
| 27 | 1 |
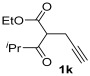
|
2a |
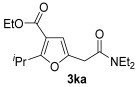
|
71 |
| 28 | 0.33 | 1k | 2a | 3ka | 60 |
| 29 | 1 |
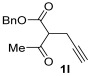
|
2a |
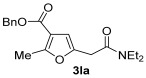
|
67 |
| 30 | 0.33 | 1l | 2a | 3la | 56 |
| 31 | 1 |
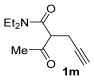
|
2a |
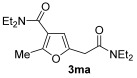
|
57 |
| 32 | 1 |
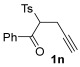
|
2c |
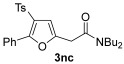
|
72 |
| 33 | 0.33 | 1n | 2c | 3nc | 67 |
a All reactions were carried out in MeCN as the solvent (0.20 mmol of 1 per mL of MeCN), in the presence of PdI2 (1 mol% or 0.33 mol%), KI (KI:PdI2 molar ratio = 100), and amine 2 (4 equiv) for 15 h, under 20 atm of a 4:1 mixture of CO–air. Substrate conversion was quantitative in all cases. b Isolated yield based on starting 1.
