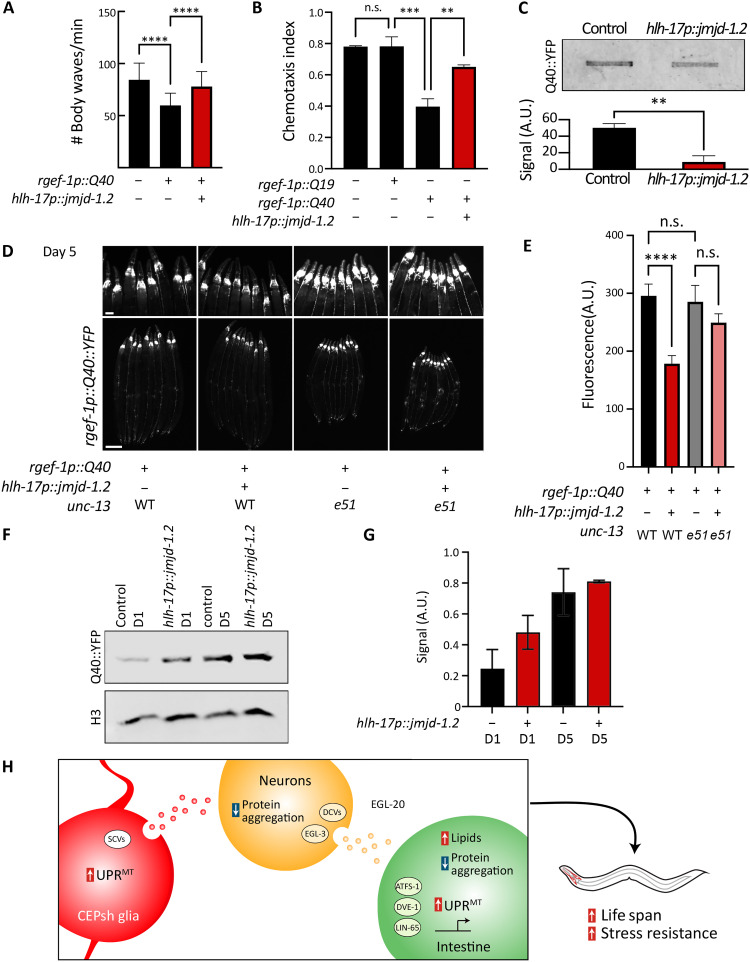
Fig. 6. Glial jmjd-1.2 rescues protein aggregation in neurons in an HD model via SCVs.
(A) Thrashing of animals expressing the aggregating polyQ tract Q40, with and without glial jmjd-1.2, measured using WormTracker (64) (n > 100). (B) Chemotaxis index of worms toward benzaldehyde (n > 200). (C) Filter retardation assay for Q40::YFP (39), with and without glial jmjd-1.2 (top) and its quantification using integrated intensity measurements in Fiji (bottom). Data are representative of three independent biological replicates. (D) Representative fluorescent micrographs of Q40::YFP for the annotated genotypes on day 5 of adulthood. See fig. S6 for images of worms at day 1. WT, animals expressing wild-type copy of unc-13; e51, animals expressing loss of function mutant unc-13(e51). (E) Integrated fluorescence intensity measurements of Q40::YFP in the head region of the animals (n > 30) using Fiji. One-way ANOVA with Tukey’s multiple comparisons test; **P < 0.01, ***P < 0.001, and ****P < 0.0001. (F) Representative blot of neuronal Q40::YFP protein at days 1 and 5 of adulthood in control (N2) and glial jmjd-1.2 (hlh-17p::jmjd-1.2) animals. Total Q40::YFP expression was measured via standard Western blots in whole worm lysates using a standard anti-GFP antibody. Signal intensity was quantified using integrated intensity measurements in Fiji in (G) and normalized to an H3 load control. Measurements in (G) were performed on two biological replicates, and data are presented as means ± SD. (H) Model of communication from glial cells to peripheral tissues. CEPsh glia use SCVs upon UPRMT activation to signal to neurons, which reduce protein aggregation and use DCVs, neuropeptide processing, and a WNT ligand to drive protein homeostasis and metabolic changes in the periphery.