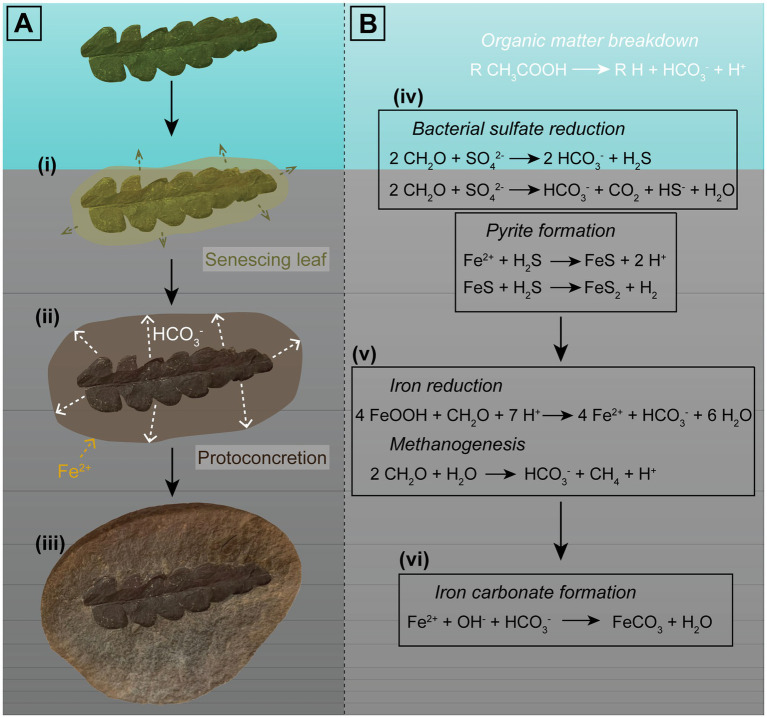
Figure 6.
Visual representation of the factors involved in formation of iron carbonate concretions in freshwater influenced environments. Sample used as an example is an iron carbonate concretion from the Mazon Creek Lagerstätte containing an Odontopteris aequalis seed fern. (A) Proposed phases of concretion growth promoted by decay of an OM source: (i) An organic nucleus, such as a leaf, is deposited near the sediment–water interface, and decay results in OM breakdown; (ii) Oxidized OM forms bicarbonate ions, which seep outwards (e.g., Yoshida et al., 2015, 2018), which could then react with Fe2+ in surrounding pore-waters. Siderite precipitation forms a ‘proto-concretion’, encapsulating the specimen and the OM; and (iii) Siderite cementation results in formation of a nodule containing a soft tissue fossil. (B) Equations representing the chemical reactions involved in OM oxidation and carbonate formation: (iv) In settings such as freshwater environments, where sulfate is limited, BSR may or may not occur. When it does, it is dependent on sulfate abundances in the pore-water and proceeds only until sulfate is consumed. The reduced sulfate will react with iron and form pyrite via iron monosulfide (e.g., Berner, 1985); (v) Once bacterial sulfate reduction ceases, OM oxidation occurs via iron reduction and methanogenesis; and (vi) This (provided conditions such as pH are suitable) promotes iron carbonate precipitation. Carbonate concretion growth is proposed to proceed until the OM is exhausted (e.g., Baird et al., 1986; Yoshida et al., 2015, 2018).