Abstract
Background
Evidence for use of second-line immunosuppressants for immune-related adverse events (irAEs) is inadequate. Therefore, a multicenter analysis should assess the efficacy of second-line immunosuppressants for severe irAEs associated with different malignant diseases.
Methods
This descriptive study aims to investigate the effects of second-line immunosuppressants on corticosteroid-refractory irAEs in patients with lung cancer. We analyzed the effects of second-line immunosuppressants on underlying lung cancer and associated adverse effects.
Results
Our study included 4589 patients who had received immune checkpoint inhibitor treatment, with 73 patients (1.6%) developing irAEs requiring second-line immunosuppressants. The most commonly observed irAE was pneumonitis (26 patients), followed by hepatobiliary disorders (15 patients) and enteritis (14 patients). We found a confirmed response rate of 42.3% for pneumonitis, which was lower than the response rates of 86.7% for hepatobiliary disorders and 92.9% for enteritis. The time from the start of corticosteroid therapy to the addition of a second-line immunosuppressant correlated significantly with the resolution of irAE to Grade 1 (correlation coefficients of r = 0.701, p < 0.005). The median progression-free survival and duration of response of underlying lung cancer from second-line immunosuppressant administration were 2.1 and 3.0 months, respectively. Of the patients with irAE, 27.4% developed infections and 5.5% might die due to infection.
Conclusion
Second-line immunosuppressant response was confirmed in 72.2% of irAEs in patients with lung cancer, with lower response rates observed in irAE pneumonitis compared to other irAEs.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00262-023-03528-x.
Keywords: Second-line immunosuppressants, Immune-related adverse events, Corticosteroid-refractory, Lung cancer
Introduction
Immune checkpoint inhibitors (ICIs), including anti-PD-1/PD-L1 and anti-CTLA-4 antibodies, have become the standard of care for lung cancer. ICIs are used for systemic therapy in advanced disease [1–3], consolidation therapy after chemoradiation therapy [4], neo-adjuvant therapy [5], and adjuvant therapy [6]. However, during and after ICI treatment, immune-related adverse events (irAEs) can occur, which are not typically seen with conventional chemotherapy and molecular target therapy [7]. IrAEs are caused by an excessive immune response not only to tumor cells but also to normal tissues, leading to organ problems and sometimes even death although severe irAEs are rare. Therefore, accurately managing irAEs is crucial in current lung cancer practice.
For moderate to severe irAEs, corticosteroids are typically used as a first-line treatment. In cases of severe irAEs not controlled by corticosteroids alone, the addition of second-line immunosuppressants, such as infliximab or mycophenolate mofetil, may be considered. These second-line immunosuppressants are expected to increase immunosuppression and resolve severe irAEs. However, evidence supporting the use of second-line immunosuppressants for irAE management has been insufficient despite clinical guideline recommendations [8, 9]. Most previous reports on second-line immunosuppressants for irAE management are single-institution retrospective analyses [10, 11], which are subject to treatment bias. Additionally, in most reports, second-line immunosuppressants were primarily used for melanoma patients although differences in the clinical course are predicted between primary malignant diseases [12, 13]. Therefore, conducting a multicenter analysis of second-line immunosuppressants for severe irAEs in patients with lung cancer would be important to establish robust evidence for their use in irAE management.
In this study, we conducted a multicenter retrospective analysis of second-line immunosuppressants for irAEs in patients with lung cancer. We analyzed the effect of second-line immunosuppressants and their adverse effect on the underlying disease.
Materials and methods
Study design and patient population
We conducted a multicenter retrospective analysis in the TOPGAN group, a Japanese lung cancer research group (TOPGAN 2022-01), with the primary objective of clarifying the effect of second-line immunosuppressants for corticosteroid-refractory irAEs. We defined steroid refractoriness as irAEs that were not controlled by steroids alone. In such cases, second-line immunosuppressants were also administered. Second-line immunosuppressants were also used to taper steroid after irAEs were under control. We collected data on patients with lung cancer who had received ICI treatment until July 2022 and had developed irAEs and received second-line immunosuppressants in addition to corticosteroids. Patient data collected included patient characteristics (age, sex, etc.), type of irAE, effect of second-line immunosuppressants, and clinical course after second-line immunosuppressant administration. Second-line immunosuppressants analyzed in this study were azathioprine, cyclophosphamide, cyclosporine, infliximab, intravenous immunoglobulin, mycophenolate mofetil, tacrolimus, and tocilizumab. This study was approved by the institutional review board of the Cancer Institute Hospital of the Japanese Foundation for Cancer Research (IRB no. 2022-GB-065), and informed consent was waived because of the retrospective nature of the study, with an opt-out option included.
Two outcomes were evaluated in this study: (1) Responses to second-line immunosuppressants determined by each investigator using patient medical records and (2) The resolution of irAEs to CTCAE grade 1 (G1) within 90 days of second-line immunosuppressant initiation. The clinical course of the underlying lung cancer was evaluated by measuring progression-free survival (PFS) and duration of response (DOR) from the initiation of second-line immunosuppressant administration. Tumor response was evaluated based on the RECIST v1.1 criteria. Incidence of infections following second-line immunosuppressant administration was also analyzed.
Statistical analysis
Categorical variables were compared using Fisher’s exact test, and continuous data were compared using the Mann–Whitney U test. The strength of the relationship between two sets of data was determined using Spearman’s rank correlation coefficient. The Kaplan–Meier method was used to estimate the median survival time. Statistical significance was set at p < 0.05. Data analysis was performed using R and SPSS Statistics for Windows version 24.
Results
Patient characteristics
Overall, 73 of 4589 patients who received ICI treatment (1.6%) developed irAEs requiring second-line immunosuppressant administration. Of the 358 patients who received combination immunotherapy with nivolumab and ipilimumab, 17 (4.7%) developed irAEs requiring second-line immunosuppressants. The frequency of patients requiring second-line immunosuppressants varied by institution, ranging from 0 to 3.2% (Fig. 1).
Fig. 1.
The frequency of patients requiring second-line immunosuppressants in each institution
The characteristics of the 73 patients who required second-line immunosuppressants are summarized in Table 1. Among them, 15 patients (20.5%) were female, 70 (95.9%) had non–small cell lung cancer, 5 (6.8%) had a history of autoimmune disease (3 with rheumatoid arthritis, 1 with dermatomyositis, and 1 with scleroderma), and 18 (24.7%) had a history of thoracic radiation therapy. Most patients (71.2%) developed grade ≥ 3 irAEs when second-line immunosuppressants were administered, and 64 patients (87.7%) were treated with corticosteroids (prednisolone ≥ 1 mg/kg).
Table 1.
Patient characteristics
| N | 73 |
|---|---|
| Age < 75 >, 75 <) | 58/15 |
| Sex (F/M) | 15/58 |
| Smoking history (current or past/never | 65/8 |
| Performance Status (0,1/2) | 67/6 |
| Histology (NSCLC/SCLC) | 70/3 |
| PD-L1 status (positive/negative/unknown) | 40/12/21 |
| Baseline prednisolone usage (10 mg >, 10 mg <) | 70/3 |
| History of autoimmune disease (positive/negative) | 5/68 |
| History of thoracic radiation (positive/negative) | 18/55 |
| Treatment line (1st line, consolidation/2nd or later line) | 50/23 |
| ICI/Chemo + ICI/ICI combination/experimental | 36/17/17/3 |
| ICI response (CR/PR/SD/PD/NE) | 3/33/19/7/11 |
| irAE grade (1/2/3/4) | 5/16/39/13 |
| Maximum steroid usage for irAE (lmg/kg >/< /unknown) | 4/64/5 |
CR complete response, PR partial response, SD stable disease, PD progressive disease, NE not evaluable
Response of second-line immunosuppressants for irAE
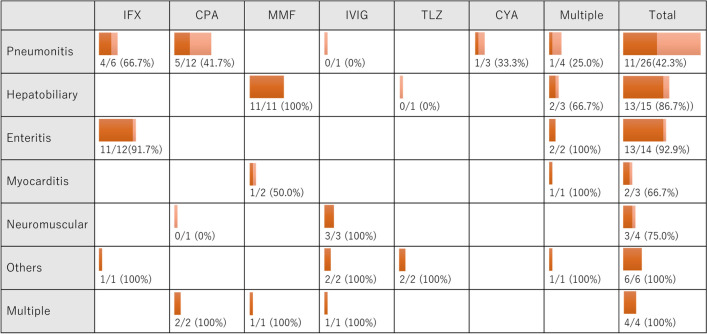
Response of second-line immunosuppressants was evaluated in 72 of 73 cases (in one case, it was unclear whether respiratory failure was due to irAE or lung cancer progression). The most common irAE was pneumonitis (n = 26), followed by hepatobiliary disorders (n = 15) and enteritis (n = 14). Infliximab was the most frequently used immunosuppressant (n = 19), followed by cyclophosphamide (n = 15) and mycophenolate mofetil (n = 14). Of the 72 cases evaluated, 52 patients (72.2%) responded to the second-line immunosuppressants. Response rates of second-line immunosuppressants in each irAE are shown in Fig. 2. Response was confirmed in 42.3% of pneumonitis cases (11/26), which was lower than the response rates of 86.7% in hepatobiliary disorders (13/15) and 92.9% in enteritis (13/14). Response based on whether the irAEs resolved to G1 or not were shown in the Supplemental material.
Fig. 2.
Response rates of second-line immunosuppressants in each irAE. Abbreviations: IFX infliximab, CPA cyclophosphamide, MMF mycophenolate mofetil, IVIG intravenous immunoglobulin, TLZ tocilizumab, CYA cyclosporine. Multiple = treated with multiple second-line immunosuppressants
Pneumonitis analysis
Among the patients with pneumonitis, 34.6% (9/26) had a history of thoracic irradiation (with definitive radiotherapy performed in all nine cases). The median time between radiation completion and pneumonitis onset was 95 (range, 31–784) days. Responsiveness to second-line immunosuppressants was confirmed in 44.4% (4/9) of cases. This was not substantially different from the 41.2% (7/17) response rate seen in those who had not undergone irradiation. The types of pneumonitis and number of cases among our patients were organizing pneumonia (OP) in 11 cases, nonspecific interstitial pneumonia (NSIP) in five cases, hypersensitivity pneumonia (HP) in one case, diffuse alveolar damage (DAD) in eight cases, and OP plus DAD in one case. The response rates to second-line immunosuppressants for each type were OP, 63.6% (7/11); NSIP, 60.0% (3/5); HP, 100% (1/1); DAD, 0% (0/8); and OP + DAD, 0% (0/1).
Correlation of timing and response of second-line immunosuppressants
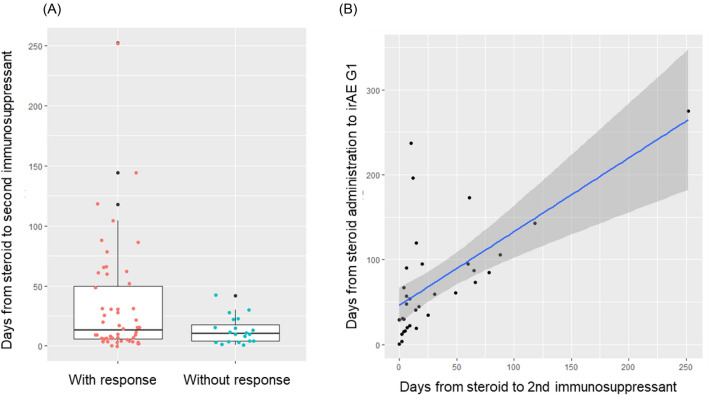
In all 72 patients evaluated, the median time from the start of corticosteroid administration to second-line immunosuppressant addition was 13.0 days in patients with a response and 10.5 days in patients without a response (p = 0.148). In the 34 patients whose irAE resolved to G1 after second-line immunosuppressant addition, there was a significant correlation between the time from the start of corticosteroid administration to immunosuppressant addition and irAE resolution to G1 (a correlation coefficient of r = 0.701, p < 0.005). (Fig. 3).
Fig. 3.
A The median time from the start of corticosteroid administration to second-line immunosuppressant addition in responder and non-responder. B The correlation between the time from the start of corticosteroid administration to immunosuppressant addition and irAE resolution to G1
Underlying lung cancer and infection after addition of second-line immunosuppressants
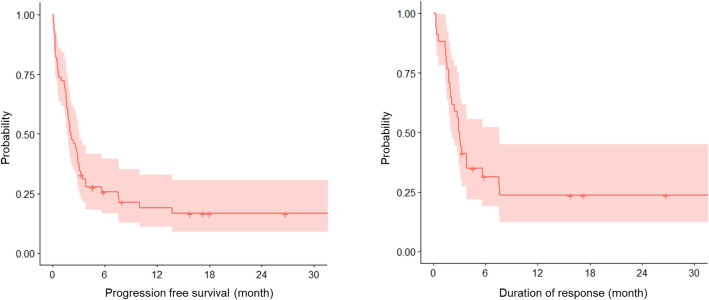
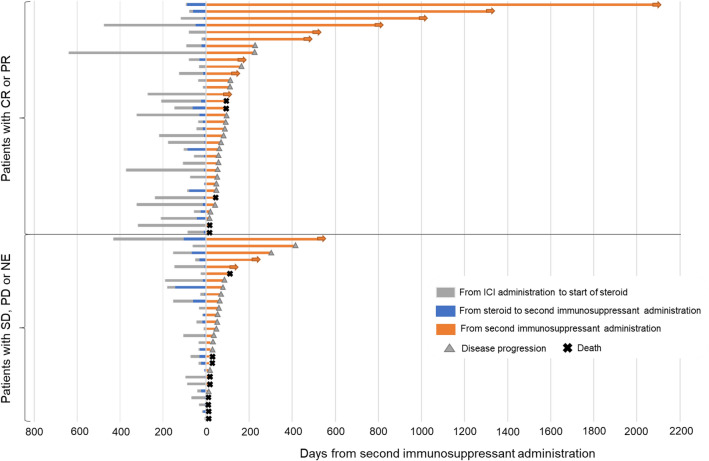
In the 61 patients who did not experience lung cancer progression in second-line immunosuppressant addition, the median PFS was 2.1 months. Among the 34 patients who remained in partial response of lung cancer in immunosuppressant addition, the median DOR was 3.0 months (Fig. 4). The detailed clinical course of each patient is shown in Fig. 5. Of the 73 patients, 20 (27.4%) developed infections after second-line immunosuppressants were added to their treatment. Ten patients had a cytomegalovirus infection, and ten had pneumonia (two cases each of pneumococcus pneumonia and pulmonary aspergillosis). The median time between the initiation of steroid treatment to the onset of infection was 46 days (range, 3–223). From the initiation of second-line immunosuppressant treatment to the onset of infection was 57 days (range, 17–272). Four patients (5.5%) might die due to infection, on top of their underlying lung cancer after the addition of second-line immunosuppressants to their treatment. The infections in these four cases were pneumonia and urinary tract infection, Legionella pneumonia, cytomegalovirus infection and pneumococcus pneumonia, and cytomegalovirus infection. The time between the initiation of second-line immunosuppressants to the onset of infection was 5, -3, 19, 39 days, respectively (In one case, infection had already occurred at the time of adding the second-line immunosuppressants). Second-line immunosuppressants were administered just once in the former two cases, while in the other two, they were continued for 15 and 42 days.
Fig. 4.
The progression-free survival and duration of response of underlying lung cancer from the initiation of second-line immunosuppressant administration
Fig. 5.
The detailed clinical course of each patient
Discussion
We collected data on patients with lung cancer who received second-line immunosuppressants for corticosteroid-refractory irAEs. The second-line immunosuppressants were used in 1.6% and 4.7% of patients who received anti-PD-1/PD-L1 and anti-CTLA-4 antibody combination therapy, respectively. The combination of anti-CTLA-4 and anti-PD-1/PD-L1 antibodies increases the rate of irAEs, which may explain the higher rate of second-line immunosuppressant usage in our study [14, 15]. We found that the response rate of second-line immunosuppressants was confirmed in 72.2% of patients, with a lower response rate observed in patients with pneumonitis (42.3%) compared to those with hepatobiliary disorders and enteritis.
A previous single-center report also showed a low response rate (30.0%) of second-line immunosuppressants in irAE pneumonitis in patients with lung cancer [10]. Other reports also demonstrated a similar tendency, where the administration of infliximab showed a response in 30% of severe irAE pneumonitis cases [11, 16]. In our report, the most commonly used second-line immunosuppressant for irAE pneumonitis was cyclophosphamide, which is different from past reports, but the response rate was similarly low. Cyclophosphamide has been reported to be ineffective in the acute exacerbation of idiopathic pulmonary fibrosis [17], and the same trend was confirmed in this study. Thus, the management and selection of second-line immunosuppressants for irAE pneumonitis need improvement compared to other irAEs.
Guidelines for managing irAEs recommend considering the addition of a second-line immunosuppressant 3–4 days after starting corticosteroid therapy [9]. A retrospective study of irAE colitis showed that early administration of a second-line immunosuppressant resulted in shorter hospitalization and symptom duration [18]. The time from the start of corticosteroid therapy to the addition of a second-line immunosuppressant correlated significantly with the time to resolution of irAE to G1 in this study. However, there was no clear correlation between the timing of the second-line immunosuppressant administration and response. Future studies should investigate the timing of second-line immunosuppressant administration, taking into account possible adverse effects, as discussed below.
The previous clinical trials have reported that patients with lung cancer who discontinued treatment due to severe irAEs tend to have a longer DOR than others [19]. However, a retrospective study showed that patients with melanoma who received second-line immunosuppressants tended to have a shorter overall survival time than others [20]. In this report, the PFS and DOR of lung cancer after second-line immunosuppressant administration were comparatively short (2.1 and 3.0 months, respectively). This indicates that excessive immunosuppression could lead to the progression of lung cancer as anti-TNF treatment has been reported to increase the risk of cancer [21]. However, no comparison has been made with a single arm, so it is impossible to draw any definitive conclusions. Moreover, in this report, 27.4% of patients developed an infection, and 5.5% of patients might die due to an infection. The adverse effects of second-line immunosuppressants may not be negligible in clinical practice.
The evidence supporting the recommendation of second-line immunosuppressants has been relatively insufficient, relying mainly on retrospective studies from single centers and expert opinions. As shown in this multicenter study, irAE management with second-line immunosuppressants is not always sufficient to resolve all irAEs, particularly pneumonitis, and adverse effects on underlying lung cancer are a concern. Therefore, it is crucial to accumulate further evidence on the use of second-line immunosuppressants in each malignant disease, including this study.
There are some limitations to this study. First, as this is a case series, we were unable to compare second-line immunosuppressants with other treatments. Second, due to the retrospective nature of this study, some biases in treatment selection and effects could not be avoided despite conducting multicenter trials to minimize these biases.
Conclusion
The response rate of second-line immunosuppressants was confirmed in 72.2% of irAEs in patients with lung cancer. However, the response rate was lower in irAE pneumonitis compared to other irAEs.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- DOR
Duration of response
- G1
Grade 1
- ICI
Immune checkpoint inhibitor
- irAEs
Immune-related adverse events
- PFS
Progression-free survival
Author contributions
RA contributed to the study conception and design. All authors made contributions to the data analysis and interpretation. SO and RA substantially contributed to the revision of the manuscript drafts. All authors reviewed the manuscript draft and revised it critically on intellectual content. All authors read and approved the final manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
T. Tozuka reports honoraria from Chugai Pharmaceuticals, Astra Zeneca, outside the submitted work, Y Tsuchiya-Kawano reports honoraria from Chugai Pharmaceuticals, Bristol Myers Squib, Astra Zeneca, Ono Pharmaceutical, Kyowa Hakko Kirin, Taiho Pharmaceutical, outside the submitted work, R. Ariyasu reports honoraria from Chugai Pharmaceuticals, Bristol Myers Squib, Astra Zeneca, outside the submitted work. The remaining authors have no conflicts of interest to declare.
Consent to participate and publish
Informed consent was waived because of the retrospective nature of the study, with an opt-out option included.
Ethical approval
This study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki and those for medical and health research involving human participants. This study was approved by the institutional review board of the Cancer Institute Hospital of the Japanese Foundation for Cancer Research (IRB No. 2022-GB-065).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 4.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N Engl J Med. 2017;377:1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 5.Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022 doi: 10.1056/NEJMoa2202170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB–IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398:1344–1357. doi: 10.1016/S0140-6736(21)02098-5. [DOI] [PubMed] [Google Scholar]
- 7.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 8.Haanen J, Obeid M, Spain L, et al. Management of toxicities from immunotherapy: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:1217–1238. doi: 10.1016/j.annonc.2022.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Schneider BJ, Naidoo J, Santomasso BD, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. JCO. 2021 doi: 10.1200/JCO.21.01440. [DOI] [PubMed] [Google Scholar]
- 10.Luo J, Beattie JA, Fuentes P, et al. Beyond steroids: Immunosuppressants in steroid-refractory or resistant immune-related adverse events. J Thorac Oncol. 2021;16:1759–1764. doi: 10.1016/j.jtho.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balaji A, Hsu M, Lin CT, et al. Steroid-refractory PD-(L)1 pneumonitis: incidence, clinical features, treatment, and outcomes. J Immunother Cancer. 2021;9:e001731. doi: 10.1136/jitc-2020-001731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verheijden RJ, May AM, Blank CU, et al. Association of anti-TNF with decreased survival in steroid refractory ipilimumab and anti-PD1–treated patients in the dutch melanoma treatment registry. Clin Cancer Res. 2020;26:2268–2274. doi: 10.1158/1078-0432.CCR-19-3322. [DOI] [PubMed] [Google Scholar]
- 13.Alexander JL, Ibraheim H, Sheth B, et al. Clinical outcomes of patients with corticosteroid refractory immune checkpoint inhibitor-induced enterocolitis treated with infliximab. J Immunother Cancer. 2021;9:e002742. doi: 10.1136/jitc-2021-002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019 doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 15.Johnson ML, Cho BC, Luft A, et al. Durvalumab with or without tremelimumab in combination with chemotherapy as first-line therapy for metastatic non-small-cell lung cancer: the phase III POSEIDON study. JCO. 2022 doi: 10.1200/JCO.22.00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beattie J, Rizvi H, Fuentes P, et al. Success and failure of additional immune modulators in steroid-refractory/resistant pneumonitis related to immune checkpoint blockade. J Immunother Cancer. 2021;9:e001884. doi: 10.1136/jitc-2020-001884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naccache J-M, Jouneau S, Didier M, et al. Cyclophosphamide added to glucocorticoids in acute exacerbation of idiopathic pulmonary fibrosis (EXAFIP): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2022;10:26–34. doi: 10.1016/S2213-2600(21)00354-4. [DOI] [PubMed] [Google Scholar]
- 18.Abu-Sbeih H, Ali FS, Wang X, et al. Early introduction of selective immunosuppressive therapy associated with favorable clinical outcomes in patients with immune checkpoint inhibitor–induced colitis. J Immunother Cancer. 2019;7:93. doi: 10.1186/s40425-019-0577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paz-Ares LG, Ramalingam SS, Ciuleanu T-E, et al. First-line nivolumab plus ipilimumab in advanced NSCLC: 4-year outcomes from the randomized, open-label, phase 3 CheckMate 227 part 1 trial. J Thorac Oncol. 2022;17:289–308. doi: 10.1016/j.jtho.2021.09.010. [DOI] [PubMed] [Google Scholar]
- 20.van Not OJ, Verheijden RJ, van den Eertwegh AJM, et al. Association of immune-related adverse event management with survival in patients with advanced melanoma. JAMA Oncol. 2022;8:1794. doi: 10.1001/jamaoncol.2022.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, Zhang Z, Wu X, et al. Risk of adverse events after anti-TNF treatment for inflammatory rheumatological disease. A meta-analysis. Front Pharmacol. 2021;12:746396. doi: 10.3389/fphar.2021.746396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.