Figure 6. Protective sGP-specific monoclonal antibodies demonstrate elevated binding affinity to sGP and neutrophil phagocytic activity.
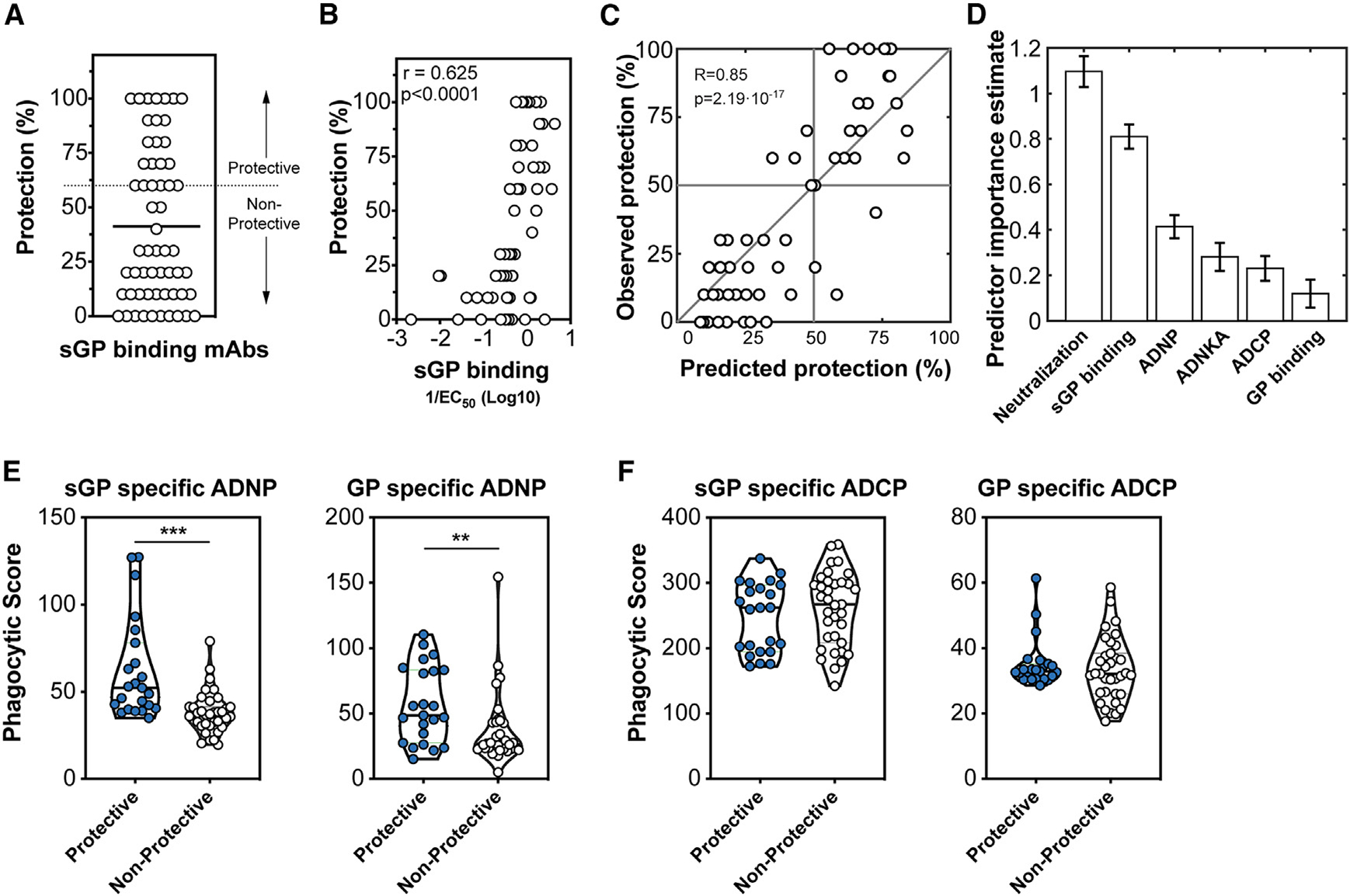
(A) Human IgG1 sGP-specific monoclonal antibodies (n = 58) collected by the Viral Hemorrhagic Fever Immunotherapeutic Consortium were evaluated for the ability to therapeutically protect BALB/c mice 2 days following infection with a mouse-adapted Ebola virus. The protection across all 58 mAbs is shown with the cutoff for protection (60%) is indicated by the dashed line.
(B) Association between the EC50 binding affinity for sGP of each antibody and protection was determined using Spearman rho correlation analysis.
(C) A model using an ensemble of decision trees, applied to antibody features, was predictive of survival as determined by leave-one-out cross validation.
(D) Features were ranked by importance using the permuted predictor delta error from 100 independently constructed models.
(E) Protective and non-protective sGP-specific mAbs were measured for the ability to induce phagocytosis of sGP-coated beads (left) or GP-coated beads (right) by neutrophils. Statistical analysis by Mann-Whitney analysis. ***p < 0.0001.
(F) Protective and non-protective sGP-specific mAbs were measured for the ability to induce phagocytosis of sGP-coated beads (left) or GP-coated beads (right) by monocytes. Statistical analysis by Mann-Whitney analysis. ***p < 0.0001.
