FIGURE 2.
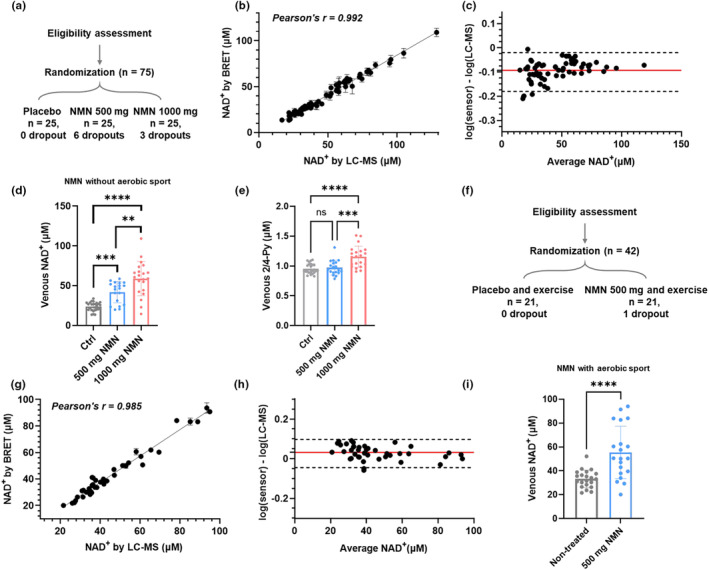
Measurement of venous NAD+ levels in clinical studies of NMN supplementation and aerobic sport. (a) Study design for evaluating effects of oral NMN supplementation. (b) Comparison of NAD+ levels measured by sensor protein and HPLC‐MS. (c) Bland–Altman analysis for venous NAD+ measured by sensor and HPLC‐MS. (d) Effect of oral NMN supplementation on venous NAD+ level. Daily supplementation of 500 mg (n = 19) and 1000 mg NMN (n = 22) for 1 month significantly increased the venous NAD+ concentration compared to the placebo group (n = 25). (e) Venous 2/4‐Py concentrations measured from blood samples with daily supplementation of placebo (n = 25), 500 mg (n = 19) and 1000 mg NMN (n = 22) for 1 month. (f) Study design for evaluating effects of oral NMN supplementation in combination with moderate level of sport. (g) Comparison of NAD+ levels measured by sensor protein and HPLC‐MS. (h) Bland–Altman analysis for venous NAD+ measured by sensor and HPLC‐MS. (i) Effect of aerobic sport with oral NMN supplementation (n = 20) and aerobic sport alone (n = 21) on venous NAD+ level. In (b) and (g), values are given as mean ± SD of three independent measurements. In (d), (e) and (i), error bars represent SD of the respective group. Significance was determined using one‐way ANOVA analysis for (d) and (e), and t‐test for (i), * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
