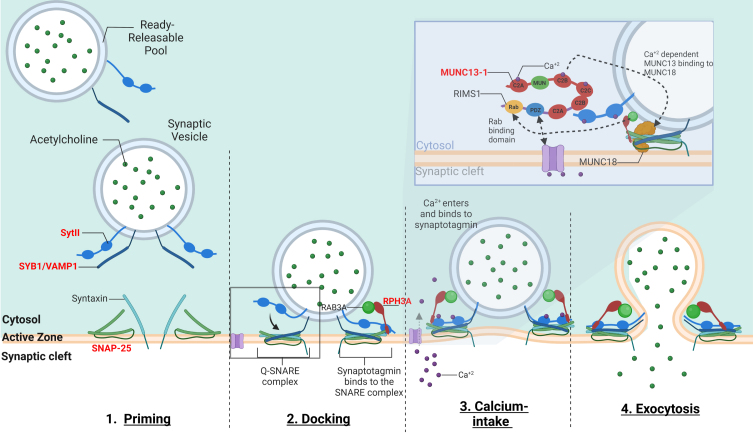
Fig. 3.
–The mechanism of calcium-dependent synaptic vesicle exocytosis at the NMJ: The ready releasable pool (RRPs) of synaptic vesicles containing ACh. When activated, the synaptic vesicle moves towards the pre-synaptic membrane terminal where calcium influx releases the synaptic vesicle bound to actin and other cytoskeletal elements (not shown in image). The “Active Zone” is the area at the pre-synaptic terminal membrane where exocytosis occurs. 1. The pool of synaptic vesicles is termed the “readily releasable pool” (RRP) and is necessary to respond to an electrical impulse. Synaptotagmin II (SytII) and Synaptobrevin/VAMP1 (SYB1/VAMP1) proteins attach to the outer synaptic vesicle membrane forming the soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptor (SNARE) complex. The cell membrane contains SNAP-25 and syntaxin components essential to the fusion of the vesicle and the cell membrane. 2. Docking –Here as the synaptic vesicle moves closer to the cell membrane, the SNARE complex begins to form 3. When an action potential depolarizes the pre-synaptic membrane, this causes the excitation of voltage-gated calcium channels and calcium entry. Calcium binds to both C2-domains (C2a and C2b) of SytII which induces a simultaneous binding of the vesicle to the cell membrane while completing the formation of the SNARE complex. Calcium induced binding of the SNARE complex requires additional proteins for vesicle exocytosis. MUNC18-1 inhibits the formation of the SNARE complex, locking syntaxin in a closed conformation, upon calcium induction MUNC18-1 interacts with MUNC13-1 opening the syntaxin conformation enabling the formation of the SNARE complex. This change in syntaxin conformation enables the binding of SYB1/VAMP1 initiating the fusion of the synaptic vesicle and cell membrane. In addition, MUNC13-1 contains C1, C2A, C2B, and C2 C domains which sequester calcium. MUNC13-1 also contains a central MUN domain. Importantly, Rab3-interacting molecule (RIMS1), which serves as an active zone scaffolding protein has been shown to interact with proteins ELKs and RIM-BPs. RIMS1 also interacts with MUNC13-1 by preventing homodimerization and recruiting MUNC13 s towards the active zone. RIMS1 also serves as a key regulator as it acts upon MUNC13 and 18, which are essential in determining the number of release sites and in the fusion of competent vesicles. RIMS1 can interact directly with the voltage-gated calcium channels at the presynaptic membrane, but altering the density of calcium channels present at the active zone, hereby effecting the size of the RRP. 4. The vesicle and cell membrane fuse together, the SNARE complex “zippers” the vesicle tightly to the cell membrane releasing ACh and other proteins into the synaptic cleft. Genes implicated in CMS are in red.