Fig. 4.
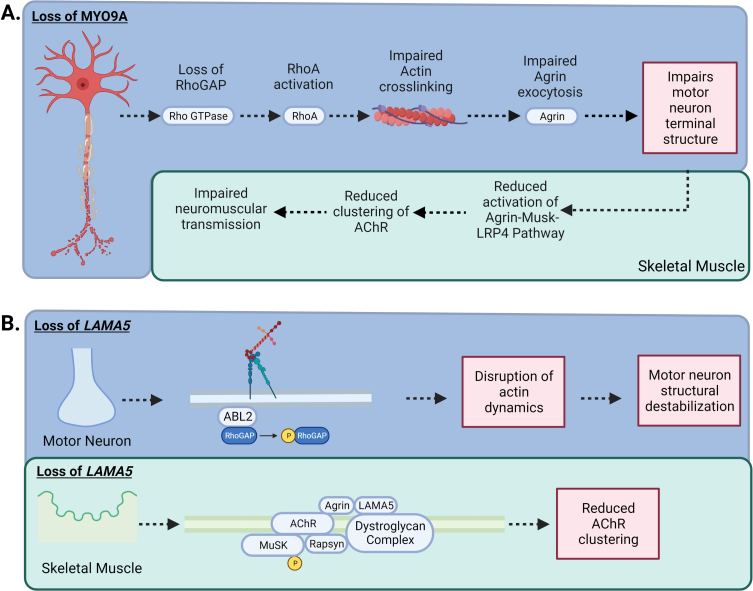
–MYO9A and LAMA5 in CMS: A) Loss of MYO9A has been shown to impair actin cross-linking, causing an alteration in the growth of axons and terminal structure. In addition, RhoGAP activity is impeded, increasing the activation of the RhoA pathway, impairing the nerve cytoskeleton and intracellular transport resulting in compromised secretion of agrin. A reduction in agrin secretion results in less activation of the Agrin-MuSK-LRP4 AChR clustering pathway. B) Laminins are the most abundant non-collagenous protein in the basal lamina. There are five extracellular matrix heterotrimeric glycoproteins composed of α, β, and γ subunits. Laminins are involved in cell signalling, development and maintenance of the NMJ, and regeneration of the nerve terminal. While Laminin α5 (LAMA5) function has not been fully elucidated, CMS patients with mutations in LAMA5 have a reduction in the number of synaptic vesicles present for release due to the small size in nerve terminals and a reduction in the number of active zones. In addition, specific mutation to the coiled-coil domain LAMA5 may play a role in preventing interaction with other laminins causing dysfunctional agrin secretion.