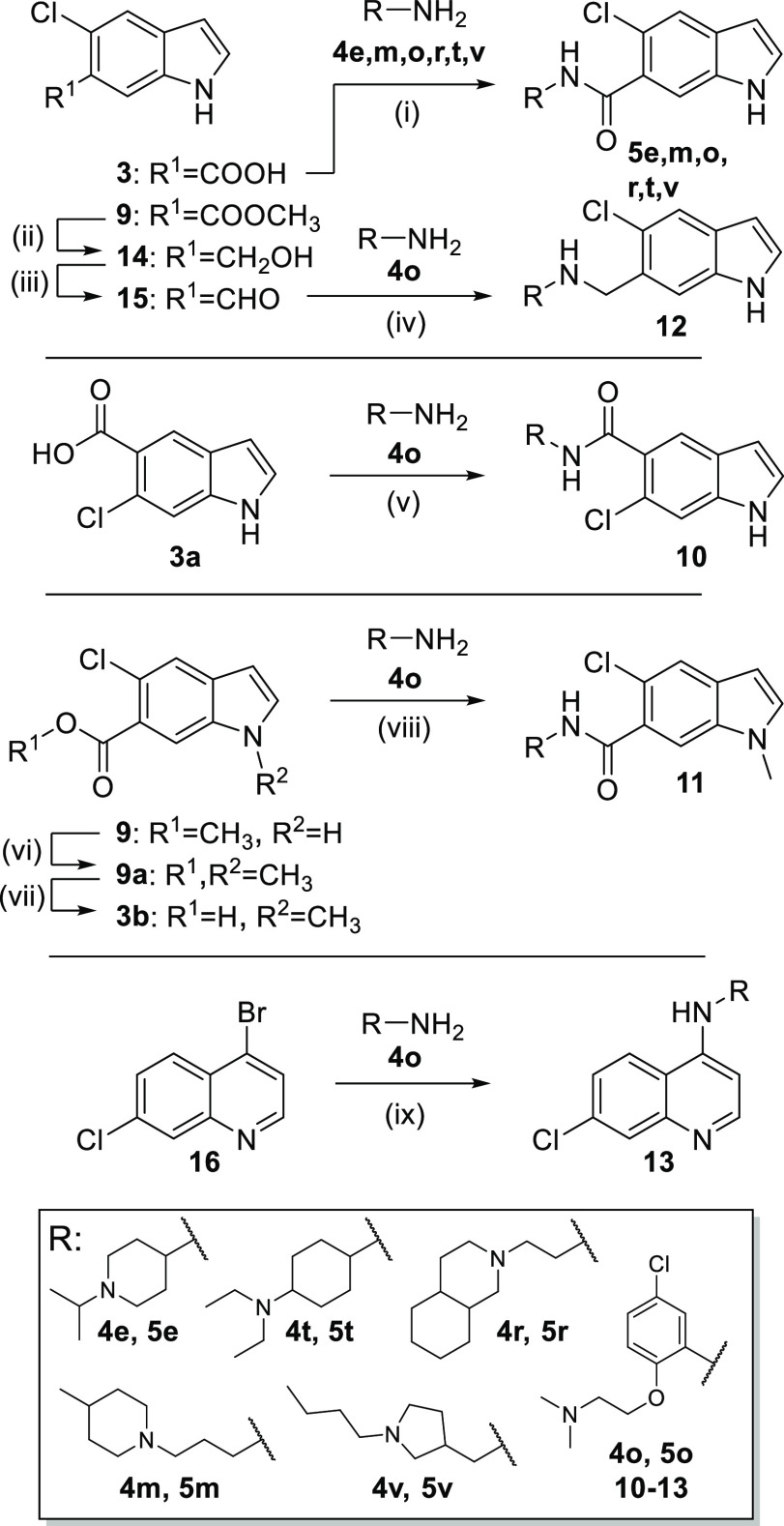
Scheme 2. Batch Synthesis of 5e, 5m, 5o, 5r, 5t, 5v, and 10–13.
Reagents and conditions: (i) EDC·HCl, TEA, CHCl3, rt, 18 h, 8–32%; (ii) LiAlH4, THF, 0 °C, 1 h, 71%; (iii) Dess–Martin periodinane, DCM, DMF, 0 °C–rt, 1 h, 100%; (iv) NaBH(OAc)3, AcOH, DCM, DCE, rt, 2 h, 36%; (v) EDC·HCl, TEA, CHCl3, rt, 18 h, 5%; (vi) NaH, CH3I, DMF, 0 °C, 10 min, rt, 2 h, 33%; (vii) LiOH·H2O, EtOH, H2O, rt, 18 h, 99%; (viii) NMI, TCFH, DMF, 80 °C, 18 h, 26%; (ix) Pd(OAc)2, BINAP, K3PO4, dioxane, 90 °C, 24 h, 29%.