Abstract
Electronic cigarette use has grown exponentially in recent years, and while their popularity has increased, the long-term effects on the heart are yet to be fully studied and understood. Originally designed as devices to assist with those trying to quit traditional combustible cigarette use, their popularity has attracted use by teens and adolescents who traditionally have not smoked combustible cigarettes. Acute effects on the heart have been shown to be similar to traditional combustible cigarettes, including increased heart rate and blood pressure. The main components of electronic cigarettes that contribute to these arrhythmic effects are found in the e-liquid that is aerosolized and inhaled, comprised of nicotine, flavourings, and a combination of vegetable glycerin (VG) and propylene glycol (PG). Nicotine can potentially induce both ventricular and atrial arrhythmogenesis, with both the atrial and ventricular effects resulting from the interactions of nicotine and the catecholamines they release via potassium channels. Atrial arrhythmogenesis, more specifically atrial fibrillation, can also occur due to structural alterations, which happens because of nicotine downregulating microRNAs 133 and 590, both post-transcriptional growth factor repressors. Liquid flavourings and the combination of PG and VG can possibly lead to arrhythmic events by exposing users to acrolein, an aldehyde that stimulates TRPA1 that in turn causes a change towards sympathetic activation and autonomic imbalance. The design of these electronic delivery devices is constantly changing; therefore, it has proven extremely difficult to study the long-term effects on the heart caused by electronic cigarettes but will be important to understand given their rising popularity. The arrhythmic effects of electronic cigarettes appear similar to traditional cigarettes as well; however, a comprehensive review has not been compiled and is the focus of this article.
Keywords: Electronic cigarette, Cardiac effects, Arrhythmia, Electronic nicotine delivery systems
1. Introduction
Electronic cigarettes, or e-cigarettes, were introduced and marketed to be used as an alternative to combustible tobacco cigarettes.1 Since their introduction in the early 2000s, e-cigarette popularity has grown among adults as a means to facilitate cessation of traditional cigarettes as well as a ‘safer’ alternative since they hypothetically emit fewer toxic chemicals in the aerosolized vapour than combustion of a traditional cigarette. However, the largest growth in use has been among adolescents due to the marketing of e-cigarettes as ‘chic’ and ‘fashionable’.2,3 Despite their introduction as a way to curb combustible cigarette smoking, young people using e-cigarettes often have no history of combustible cigarette use, termed ‘never smokers’.1 Adolescents report curiosity, peer-pressure, and appealing flavours as reasons for e-cigarette initiation.1 Another contributing factor to increased adolescent interest is the targeting of youth by e-cigarette companies through ads on social media.4 In contrast, the intent to cease combustible tobacco cigarette use is the main reason for e-cigarette use among adults, yet some of the youth and adult population claim to have not smoked combustible cigarettes previously.5
Although there has been a notable decrease in combustible cigarette use in the past decade, the overall use of tobacco among the youth population does not follow that same trend. This could be due to other methods of tobacco use increasing in popularity, including, but not limited to, e-cigarettes.6 According to the 2009 National Youth Tobacco Survey (NYTS), 23.2% of high school seniors reported to have smoked a cigarette in the last 30 days, while the 2021 NYTS reported 43.6% of high school students reported using e-cigarettes on 20 or more of the past 30 days and 27.6% reported daily use.7,8 The Centers for Disease Control and Prevention (CDC) also reported that 2.55 million school-aged adolescents reported e-cigarette use in the year 2022, and of those who reported, it is estimated that 85% of them used e-cigarettes containing flavourants.9 Although adolescent cigarette use has declined, tobacco use as a whole has increased largely as a result of the increasing popularity and access to flavoured e-cigarettes.
E-cigarettes can be considered a valid alternative for combustible cigarettes, but the evidence lacks on whether or not they can be considered safer. Despite the promotion of e-cigarettes as a ‘safer’ combustible cigarette alternative, tobacco use remains a global health burden. According to a 2019 study, among those who were considered current smokers, 89% of their deaths were attributed to their use of smoking tobacco.10 In 2022, the CDC reports an estimated 7 million deaths can be attributed to tobacco use per year; a statistic that will rise to 8 million deaths within the next 10 years due to the lasting effects of smoking tobacco.11 The unprecedented occurrence of a global pandemic has also affected the rate of combustible cigarette use. According to a study sampling from current and former smokers, the rate of smoking during the pandemic increased, which can be attributed to increased stress experienced during that time.12 A majority of the studies focusing on the effects of e-cigarettes on systems in the body focus on short-term effects. In this regard, e-cigarettes have the potential to be safer than combustible cigarettes. However, the amount of clinical studies investigating the long-term effects of e-cigarette use is significantly limited.13
Most e-cigarette devices are composed of three main elements: a source of power, a heating element, and a liquid or solvent that is aerosolized and inhaled.2 Nicotine and aerosol constituent yield, or the amount of aerosolized solvent emitted from the e-cigarette and inhaled by the user, can be influenced by factors such as user behaviour, voltage, and liquid ingredients.1 Usage varies among factors such as puff volume and duration of puff, with a longer puff resulting in a higher nicotine yield.1 The voltage in an e-cigarette can vary depending on the battery or power source;1 not only can increasing the power of the e-cigarettes increase the yield of nicotine, but it also can increase the number of toxicants emitted from the device.1,14 Glycols, aldehydes, metals, and volatile organic compounds are examples of such harmful materials.1 The aerosolized vapour can differ in composition but is usually a mixture of nicotine, a flavourant, and a solvent, typically made up of propylene glycol (PG), vegetable glycerin (VG) or a combination of the two.1
The ratio of the PG/VG solvent is an important factor when considering nicotine yield). These solvents differ in boiling point, as well as aerosol particle size.1 Other aspects of the liquid, such as pH, must also be taken into consideration when determining aerosol constituent yield and, more specifically, the form of nicotine. Depending on the pH, nicotine can either be in its free-base or protonated forms.14 One study found that although the form of nicotine did not influence its yield, it did influence nicotine delivery to the blood.14 There has been limited investigation on the impact of flavourants on aerosol constituent yield;1 however, these flavourants may be responsible for the increase in e-cigarette popularity among youth.
While proposed as a way to combat the toxic effects of combustible cigarette use, e-cigarettes present problems of their own. The use of e-cigarettes has impacts on many systems in the body including the cardiovascular, respiratory, and nervous systems.1 Studies indicate that short-term use of e-cigarettes contributes to mild acute effects, but the potential long-term effects remain unknown due to a limited amount of research and since they have only been on the market since the early 2000s.15,16 Examples of such acute effects include negative effects on arterial stiffness, risk of thrombogenesis, high levels of inflammatory cytokines, and increased oxidative stress.16 A recent study indicated that short-term exposure to e-cigarette use increased angiogenesis in mouse heart tissue.15
2. E-cigarettes and their components
E-cigarettes have grown to be the most popular form of nicotine delivery devices in the United States; the current (4th) generation e-cigarettes, which are known as ‘pod-mods’ or ‘pod-mod’-like disposable e-cigarettes are the most popular type of e-cigarette, especially among adolescents. A national youth tobacco survey found that 72.4% of student e-cigarette users reported the use of 4th generation e-cigarettes.17 These latest forms of e-cigarettes are characterized by their new sleek, portable design, and a more enjoyable user experience as a result of appealing flavours and increased nicotine concentrations compared to prior generations.18
There is a widely held belief that e-cigarettes may be safer than traditional cigarettes due to lower levels of harmful substances that are linked to cardiovascular and pulmonary issues. However, numerous studies have determined that both acute and chronic use of e-cigarettes can be detrimental to the body. Studies have demonstrated that during short-term exposure, human subjects demonstrate elevated blood pressure and heart rate (HR), similar to the effects seen with conventional cigarettes.19 Additionally, prolonged exposure in mice has been shown to result in more severe effects, including increased sympathetic activity, increased arterial and vascular stiffness, impaired endothelial function, angiogenesis, and the formation of atherosclerotic plaque formation.20 These effects are a result of exposure to a combination of components of e-cigarettes including solvents, flavourings, and nicotine. However, it is crucial to also assess the health risk posed by each of these individual components.
2.1. Nicotine
Nicotine is a chemical used in e-cigarettes and other forms of nicotine delivery systems that acts as the main contributor of psychoactive and addictive effects on users. The presence of nicotine in e-cigarettes plays a major role in the development of negative cardiovascular and arrhythmic effects in users. E-cigarette use without nicotine does not produce the same detriments.21 Nicotine is absorbed in the mouth and lungs, where it enters the pulmonary venous circulation and passes through the heart, before entering the arterial circulation and entering the brain.22 After the nicotine crosses the blood-brain barrier and enters the brain, it binds to nicotinic acetylcholine receptors (nAChRs) which, in turn, activate a signal transduction pathway that releases neurotransmitters such as dopamine, norepinephrine, serotonin, GABA, glutamate, and endorphins.22 The release of dopamine in the brain signals a pleasurable experience and increases brain reward function. Withholding nicotine leads to decreased amounts of dopamine, which can further lead to reduced brain reward function and is an important part of nicotine addiction.23 The release of these neurotransmitters, especially dopamine, in the brain facilitates the addictive aspects of e-cigarettes, while also contributing to arrhythmic effects that also occur in traditional cigarette smokers.
Nicotine also induces various vascular effects, including constriction of blood vessels, increased HR, increased blood pressure, increased myocardial contractility, fibrosis, endothelial dysfunction, oxidative stress, and arterial stiffening.19,24 Although the specific mechanisms in which these effects occur are not fully understood, they likely begin in a similar manner to the effects that occur in the brain, with nicotine stimulating the sympathetic nervous system. One study found that when users who used e-cigarettes with nicotine had significant increases in pulse wave velocity, aortic pulse pressure, augmentation index corrected for HR, and sub-endocardial viability ratio, while the e-cigarettes without nicotine did not elicit the same effects.25 Other studies have shown that e-cigarettes without nicotine are capable of increasing aortic pulse wave velocity and resistivity index, which makes it unclear as to whether or not nicotine is solely responsible for the vascular effects, and shows that other factors like the e-cigarette itself, and the other e-liquid constituents also play a role in inducing these effects.26
2.2. Nicotine salt vs. free-base nicotine
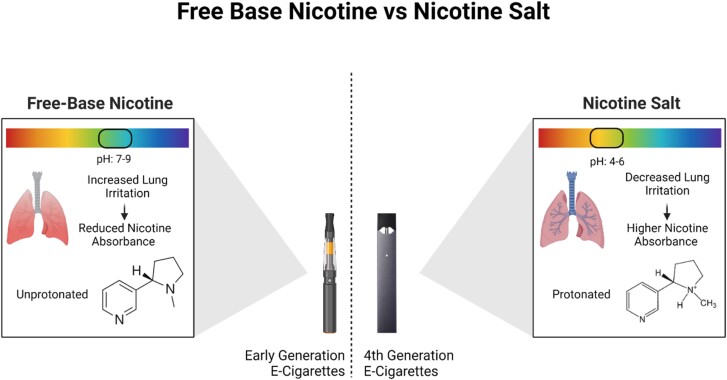
Early generation e-cigarettes used free-base nicotine in their e-liquid, which is nicotine in its unprotonated form with a pH of around 7–9. Free-base nicotine is harsher to inhale, so the nicotine levels in early generation e-cigarettes were generally lower. Free-base nicotine is also lipophilic, which means it can diffuse across membranes and can increase bioavailability via lung absorption, whereas protonated nicotine salt is hydrophilic and membrane diffusion does not readily occur.17 The pod-mod and pod-mod-disposable e-cigarettes have incorporated salt-based nicotine, which produces a more acidic pH that lessens the harshness and irritation with inhalation (as shown in Figure 1).27 With the decreased harshness, newer generation e-cigarettes are able to increase their nicotine concentrations, thus furthering the adverse health effects. A study on one of the most popular pod-mod e-cigarettes, JUUL, showed that vaping one 5% pod was the equivalent of up to 30 cigarettes, depending on factors such as the flavour and user behaviour.27
Figure 1.
Comparison of the pH and inhalation effects of free-base vs. salt-based nicotine. Free-base nicotine has a higher pH that leads to increased lung irritation and in turn, reduced nicotine absorbance. Salt-based nicotine has a lower pH that leads to decreased lung irritation and higher nicotine absorbance.
2.3. Nicotine yield
Factors that influence nicotine yield include the concentration of nicotine itself, the power of the e-cigarette, the pH of the e-liquid, user behaviour, and PG/VG ratio.28 Bioavailability of nicotine is largely influenced by the pH of the inhaled aerosol, and the pH is directly influenced by the concentration of the nicotine in the e-liquid, with higher concentrations of nicotine leading to higher pH.22 Nicotine is a weak base, which means at a higher pH, a large amount of nicotine is present in a gas phase of the aerosol that is inhaled; the increased presence of nicotine in its gaseous phase leads to increased buccal and upper respiratory tract absorption, but also comes with a more unpleasant taste and increased irritation during inhalation. At a lower pH, more nicotine is present in a liquid phase of the inhaled aerosol, which creates a more pleasant taste from the aerosol. In turn, this allows the user to inhale deeper and longer, thus influencing user behaviour. The ability to inhale deeper causes increased lung deposition of the aerosol and increased nicotine absorption in the lungs.22 With the newer generation of e-cigarettes predominately using salt-based nicotine, the two most variable factors that influence the yield are the concentration of nicotine and user behaviour. Higher concentrations of nicotine, longer puff durations, and larger puff volumes all lead to more nicotine entering the bloodstream.
2.4. Liquid flavourings
Flavourings contained in e-cigarette liquids have driven e-cigarette demand, especially among secondary school-aged students.29 In one study, 97% of middle and high school-aged e-cigarette users report using a flavoured product, while 70% of these users claim flavourings to be their reason for use.30 These flavourings are mixed in the liquid cartridge along with other solvents and chemicals such as PG, VG, and nicotine. The most common flavours among users are fruity, tobacco, desserts, drinks, candies, and menthol.31 While PG, VG, and nicotine can cause significant damage to multiple organ systems in the body, the common additive flavourings are just as harmful to the cardiovascular system. While these flavourings are approved by the Food and Drug Administration (FDA) for consumption, they are not approved for inhalation and heating of the flavouring-containing solvent releases chemicals in addition to those released by heating PG/VG and/or nicotine.32 Increased concentration of flavourings is associated with increased production of aldehydes including formaldehydes, acrolein, and vanillin, indicating that flavouring compounds are a key source of toxic aldehyde production. Furthermore, these compounds are not part of the mixture of flavouring compounds, and are instead formed during chemical reactions of the e-liquid constituents during heating.33
2.5. Solvents
The final component of e-liquid is the solvent, which plays a dual role in diluting the nicotine to the target concentration and giving the liquid a consistency that allows it to be easily vapourized.34 Exposure to aerosolization products of VG has been associated with eye, lung, and oesophagus irritation; PG exposure has been correlated with symptoms of upper respiratory infection. The ratio of the two solvents in the liquid is capable of forming reactive oxygen species, which have been linked to cardiovascular diseases such as arteriosclerosis, hypertension, restenosis, and ischaemia.35–37 Early generation e-cigarettes, while displaying similar reactive oxygen species reactivity profiles to traditional cigarette filters and smoke, also output 6.1 times more copper per puff than reported for traditional cigarettes.38 Along with the formation of reactive oxygen species, the ratio of PG to VG also plays a large role in the delivery of nicotine. In a recent research study on pod-mod and pod-mod-disposable e-cigarettes, all 4th generation e-cigarettes use a combination of both solvents ranging from 80PG:20VG to 50PG:50VG.39 An increased ratio of PG in the e-liquid results in higher amounts of nicotine delivery to the user.28 Other studies have shown additional relationships between the ratio and nicotine delivery, but since the ratio changes depending on the brand of e-cigarette, and often the actual ratio is different than the listed ratio, the direct relationship between PG:VG and nicotine yield is difficult to study.
2.6. Battery
The battery of the electronic cigarette plays a role in e-liquid delivery as a result of varying levels of voltage and resistance that alter the power, which can affect how the e-liquid and its constituents are exposed to the user. Power of an e-cigarette can be calculated by dividing the square of the voltage by the resistance.39 In older generation e-cigarettes, users were able to customize certain settings, like the voltage, to affect the power, but that feature is not common in current generation e-cigarettes. The power of an e-cigarette can affect the total nicotine emission, total particular matter, and the amount of carbonyls produced by an e-liquid after vapourization. A study using various pod-mod-like disposables showed that higher power tends to lead to higher nicotine emission, higher total particulate matter emission, and higher emission of carbonyls, but variables such as nicotine concentration, PG/VG ratio, brand, and flavour can also play a role in affecting these results, so more research needs to be done to truly understand the effects of battery power.39
3. Arrhythmias induced via e-cigarettes
Arrhythmias can appear in various forms, such as inappropriate slowing or accelerating of the HR, termed bradycardia and tachycardia, respectively.40 Bradycardia, tachycardia, and abnormal HR variability heart can be caused by dysfunction in the sinoatrial node, or can be secondary to another condition, disease, or disorder.41 Atrial and ventricular cells can also depolarize out of rhythm and cause arrhythmias due to dysfunction in sodium currents (INa), calcium currents (ICa or INCX), and/or potassium currents (IKr, IKs, IK1, Ito, etc.), causing atrial and ventricular arrhythmias.42 Fibrosis, typically characterized by the abnormal accumulation of extracellular matrix proteins, is also predictive for propensity to arrhythmias by perturbing normal action potential conduction.43 Atrial fibrillation (AF), a type of atrial arrhythmia, is the most common type of arrhythmia in the world.40 In 2019, AF and atrial flutter were estimated to affect 59.7 million people worldwide, which is double the number of cases in 1990.44
3.1. Clinical evidence of pro-arrhythmogenic markers associated with e-cigarette use
The current literature on e-cigarettes is widely varied and sparse on information due to the ever-changing landscape of e-cigarette technology and use. Therefore, many current hypotheses about e-cigarettes and their arrhythmogenic effects are relegated to pre-clinical animal studies. However, a handful of studies have shown some epidemiological evidence between e-cigarette use and pre-arrhythmogenic markers. One systemic review of 19 studies found e-cigarette use to increase sympathetic excitation, which translated into increases in heart rate variability (HRV), HR, and blood pressure, which they attributed to the nicotine (rather than other non-nicotine constituents in e-cigarettes). However, sympathetic excitation was not as dramatic as in traditional cigarette smokers.45 Another study analysed the Fast Fourier transform components of HRV in habitual e-cigarette users and compared them to non-e-cigarette users. Habitual users generally had decreased high-frequency (HF) component, increased low-frequency (LF) component, and increased LF/HF ratio of HR when measured after not using e-cigarettes for at least 12 h; all are consistent with increased sympathetic activity.46 Low-density lipoprotein oxidation, a common measure of oxidative stress, was also elevated in habitual e-cigarette users.38 This increased sympathetic activity has been shown to result in increased propensity to arrhythmias in traditional cigarette smokers.39,40 However, one recent study that specifically explored the acute effects of 4th generation e-cigarettes in habitual users, although still identifying increased sympathetic tone via increases in blood pressure and HR, reported decreases in HRV acutely after e-cigarette use.47 They also reported other negative indicators of cardiovascular and pulmonary health such as reduced exercise capacity 90 min following e-cigarette use and reduced 60 s HR recovery. A 60 s HR recovery of less than 25 beats per minute (bpm) after exercise increases the risk of sudden cardiac death by a factor of 2.2.48
3.2. HR and HRV
Exposure to aerosol from e-cigarettes in mice decreased HR, termed bradycardia, and increased HRV, the variation of time between consecutive heartbeats.49 HRV is a useful index in cardiovascular disease and can be a prognostic tool for poor clinical cardiac outcomes when abnormally high or low.50–53 HR is modulated by both the sympathetic and parasympathetic nervous systems, and therefore, abnormal HRV can indicate imbalance in sympathetic and parasympathetic tone.51 Exposure in male mice in nicotine-containing aerosols with just flavourings (no PG or VG) experienced significant but smaller changes in HR and HRV. Bradycardia in response to PG/VG solvent was apparent after just five puffs, but HR began to recover immediately following exposure.49 Male mice experienced more significant bradycardia, with decreased HR by a mean of 300–400 bpm upon first exposure interval to PG/VG aerosolized solvent. Female mice generally experienced about a 100 bpm decrease in HR upon first exposure interval in response to PG/VG.49 However, another study identified decreased HRV in 8–10-week-old rats of both sexes when chronically exposed to JUUL e-cigarette aerosol for 5 min per day over 2 months alongside increased sympathetic innervation.54 Another mouse study published similar findings after chronic exposure to e-vapour for 2 h per day over 10 weeks, which indicates that chronic e-cigarette exposure likely causes long-term sympathovagal disbalance.55 Interestingly, these numerous animal studies exploring HR and HRV effects of e-cigarette aerosol exposure consistently find acute decreases in HR, which is in stark contrast to many of the clinical studies.
3.3. Ventricular arrhythmogenesis from nicotine
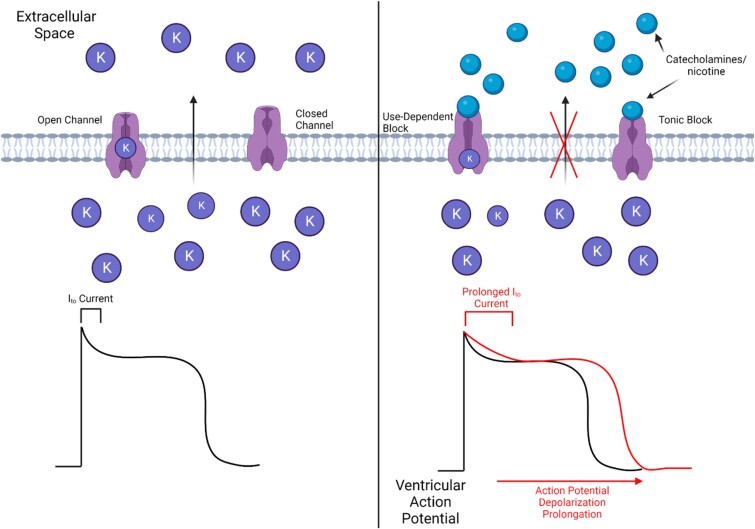
Along with the release of various neurotransmitters, the binding of nicotine to the nAChRs stimulates the release of catecholamines, which itself can play a role in causing arrhythmogenic effects in the ventricle.56 The release of catecholamines and direct interaction of nicotine with channel proteins blocks the cardiac inwardly rectifying potassium K1 current (IK1), which is generated by Kir 2.1 and 2.2 channels, inhibiting the inward flow of potassium ions.56,57 Along with IK1, nicotine can block the cardiac rapid delayed rectifying potassium current (IKr), which is generated by Kv11.1 (or hERG) channels, even without the involvement of catecholamines, but at nicotine levels higher than realistic concentrations such as 1–50 µM, of nicotine in the blood.58 However, the inhibition of the cardiac transient outward potassium current (Ito) by nicotine is relevant to the amount of nicotine in the blood of high-nicotine users. Nicotine inhibits Ito current and the Kv4.3 channel by blocking the outward flow of potassium ions via tonic and use-dependent blocking (as shown in Figure 2).59 The blocking of Ito causes a depolarization of the resting potential and a delay of the late phase of membrane repolarization as a result of the change in concentration in potassium ions, thus affecting the normal rhythm of the heart and inducing arrhythmic effects.
Figure 2.
Depiction of the potential effects of interactions of catecholamines and nicotine with K+ channels on ventricular action potentials. Catecholamines and nicotine block potassium channels via use-dependent, which is the block of an open channel, and tonic block, which is the block of an already closed channel. The blocking of these channels leads to a prolonged Ito current and subsequent action potential depolarization prolongation.
Adverse alterations in ventricular repolarization can be an indicator of ventricular arrhythmogenesis, with these changes resulting from nicotine entering the blood circulation, increasing the workload and oxygen demand of the myocardium.60 Abnormal Tp-e interval, the interval between the peak of the T-wave and the end of the T-wave, and Tp-e/QT ratio (QT is the interval between the beginning of the Q wave to the end of the T-wave), were observed in e-cigarette users, which have been said to be electrocardiographic indicators of irregular dispersion of ventricular arrhythmogenesis and sudden death.60 Observations of these electrocardiographic characteristics have been reported on people with Brugada syndrome, a condition that increases the risk of irregular ventricular heart rhythms. Electrocardiograms can help determine arrhythmogenic effects of e-cigarettes by comparing the Tp-e interval and the Tp-e/QT interval of the two. Exposure to e-cigarette aerosols increases ventricular arrhythmia, measured as ventricular premature beats.49 Prenatal nicotine exposure in rats resulted in higher ventricular arrhythmias (premature ventricular contractions) at 4–5 months of age, which was exacerbated in response to adult nicotine exposure.61
3.4. Atrial arrhythmogenesis from nicotine
The molecular and electrophysiological mechanisms that cause atrial arrhythmogenesis are similar to those that cause ventricular arrhythmogenesis, but the atria are also subject to structural alterations that are likely to cause AF, a type of atrial arrhythmia. Similar to what occurs in ventricular arrhythmogenesis, the blocking of K+ channels affects the electrophysiology of the atrium, causing it to become unstable as a result of the influence on the cardiac repolarization and membrane transport processes.62 The structural mechanism of atrial arrhythmia has been studied in canine models and is characterized by the severe slowing of local conduction and heterogeneous electrical activities associated with structural abnormalities with prominent fibrosis between and within atrial muscle bundles.63 These structural alterations make the atria more susceptible to AF, as a result of nicotine downregulating microRNAs 133 and 590. The production of miR-133 and miR-590 act as post-transcriptional repressors of transforming growth factor (TGF) Beta1 and TGF-Beta, so the downregulation of miR-133 and miR-590 causes an increase in growth factors TGF-Beta1 and TGF-Beta receptor type II, which regulates the production and deposition of collagen.63
Various factors negatively affect the ability of researchers to determine the specific chronic effects, specifically cardiac structural remodelling, of vaping in humans, but pre-clinical evidence found that chronic exposure to e-cigarette aerosol is capable of forming multi-organ fibrosis in the heart, kidneys, and liver.64 In this experiment, mice were exposed to e-cigarette aerosol daily, for 5 days a week, for 3–6 months. Heart tissues were assessed with Masson’s trichrome staining and identified a 2.75-fold fibrosis increase (P < 0.05) in the C57BL/6 mice, which were known to be susceptible to emphysema.64 The study was done to best mimic human use and they determined that humans using e-cigarettes (with PG, VG, and nicotine), could be at risk of developing fibrosis. Although there is still much work that needs to be done before this effect is fully understood, there is reason to be concerned about cardiac fibrosis as a result of e-cigarette use.
Male mice exposed to repeated e-cigarette aerosol puffs containing either PG, VG, PG/VG mix, e-tobacco, or e-menthol (91 mL puff, 2 puffs/min, 18 puffs/session) experienced prolonged P-wave duration during exposure. PG/VG (286.7 mg) and menthol (191.9 mg) exposure (9 min) also resulted in prolonged PR interval during exposure. Male mice also experienced supraventricular block (non-conducted P-waves) during e-cigarette aerosol exposure, which were especially pronounced in response to PG/VG and VG aerosols. However, P-wave and PR interval durations were both generally shortened within the following 5–30 min post-exposure to the various e-cigarette aerosols, indicating an acceleration in supraventricular conduction. These data indicate that the individual components of e-cigarette aerosol cause supraventricular conduction defects during and after exposure.49 Conduction defects play a significant role in AF initiation and propagation. Sources of AF within the heart are generally found near conduction blocks.65
3.5. Liquid flavourings and arrhythmia
Aldehyde exposure from liquid flavourings in e-cigarettes is known to elicit various arrhythmia conditions. Researchers have discovered that increasing formaldehyde vapour levels in rat models can induce profound bradycardia and non-sustained polymorphic ventricular tachycardia.66 Acrolein exposure, also known as propylene aldehyde, increases HR variability, occurrence of arrhythmias, and left ventricular developed pressure in mice models through TRPA1 activation and autonomic imbalance.67 Mice exposed to acrolein (3 ppm for 9 min) experienced a moderate yet significant decrease in HR and increased HRV when exposed to acrolein. Post-exposure, mice experienced QTc prolongation, signifying alterations in ventricular repolarization in response to acrolein (as shown in Figure 3).49
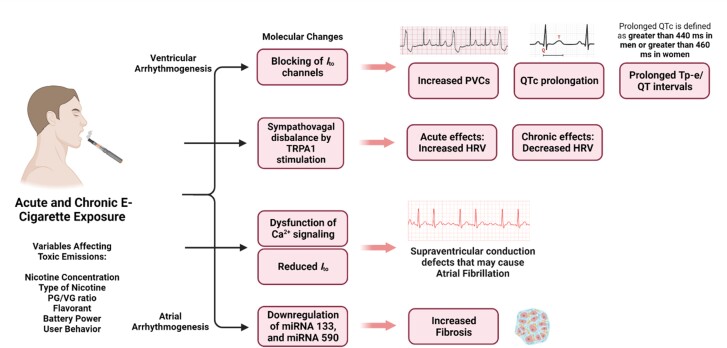
Figure 3.
Various arrhythmogenic effects known to occur as a result of e-cigarette use. The reduction and blocking of Ito channels, sympathovagal disbalance, Ca2+ signalling dysfunction, and downregulation of certain miRNAs all lead to arrhythmogenic effects in e-cigarette users. Which effects are felt and the extent to which they are experienced can be determined by e-cigarette characteristics that alter toxic emissions, such as the nicotine concentration, type of nicotine, PG/VG ratio, flavourants, battery power, and user behaviour.
The presence and combustion of PG and VG also contribute to the production and inhalation of toxic carbonyl compounds, including formaldehyde, acetaldehyde, and acrolein. However, the extent of this production is strongly dependent on the power levels of the e-cigarette device. Of the toxic compounds that are inhaled, acrolein plays the most significant role in increasing the risk of experiencing arrhythmic effects in users; the increase in arrhythmic susceptibility was shown in rats that were exposed to diesel exhaust, an air pollutant that contains acrolein.68 The research that was done on these rats suggests that the arrhythmic response came as a result of acrolein stimulating TRPA1, a chemosensor that is located on airway sensory nerves, causing a change towards sympathetic activation and autonomic imbalance.68 Similar to the effects that nicotine has on the heart, the activation of TRPA1 affects the cardiac electrical conduction system, potentially via the alteration of thresholds, which can in turn increase the risk of experiencing arrhythmic effects after inhalation of significant amounts of acrolein.68 Additionally, aldehydes in vanilla and apple jax flavours such as vanillin and cinnamaldehyde are associated with cardiac electrophysiological instability and inducible arrhythmogenesis in mice independent of nicotine and solvent concentrations.55
3.6. Particulate matter exposure and arrhythmia
E-cigarette aerosol is known to contain both fine and ultra-fine particulates.69 As shown above, e-cigarette exposure can have dramatic effects on both heart function and arrhythmogenesis, similar to particulate matter (PM)-induced effects on the heart.70 An additional factor in the induction of arrhythmic effects is the production of fine and ultra-fine particles—particles that have a diameter of 2.5 µm or less. Studies have shown that e-cigarette users are exposed to significant and unhealthy amounts of these particles.71 The cardiovascular effects that are caused by these particles are achieved through two pathways: an indirect and a direct pathway. In the direct pathway, the particles enter the bloodstream directly and expose users to an increased risk of developing cardiac arrhythmias by increasing the concentration of intracellular calcium.72 The indirect pathway is characterized by the deposition of the particles in the lungs, which causes development of inflammatory responses and inflection of the autonomic nervous system; the modulation of the autonomic nervous system causes an increase in vasoconstriction and change in HRV, which has the potential to increase the risk of experiencing arrhythmic effects.72 However, studies have yet to show a direct relationship between e-cigarettes, particulate matter, and arrhythmias, so we have extracted findings based on combustible cigarettes since e-cigarettes produce a similar amount of PM.
4. Traditional combustible cigarettes
Numerous studies have shown an association between AF and traditional combustible cigarette smoking. In older populations (55 years of age or older), smokers had more than a 50% increase in AF risk than non-smokers.73 Multiple other studies have identified an AF hazard ratio of 1.41–2.05 in cigarette smokers compared to never smokers.74–76 Not only have studies identified propensity to AF in smokers but also an increase in AF in the offspring of smokers. For every pack/day of parental cigarette smoking, the child experienced an 18% increase in likelihood of experiencing AF.77 Even after catheter ablation to treat AF, cigarette smokers with pulmonary vein plus right atrial non-pulmonary vein triggers had higher rates of recurrence.78 Cigarette smoking has also been associated with increased atrial fibrosis, which is a substrate for atrial arrhythmia.79 A common measurement of ventricular repolarization, the Tp-e/QT ratio measured via electrocardiogram, has been associated with ventricular arrhythmias when prolonged.80 Heavy smoking has been associated with prolonged Tp-e/QT intervals.81 Patients with ischaemic left ventricular dysfunction that smoked had increases in ventricular arrhythmias.82 HRV, which some suggest as a precursor for arrhythmia, is shown to increase in smokers, particularly within the first 5–10 min of smoking traditional combustible cigarettes.83 Beat-to-beat alternations in systolic Ca2+ transient amplitude, defined as cardiac alternans, are markers for susceptibility to severe ventricular arrhythmias and sudden cardiac death.84 In male mice, secondhand smoke at levels relevant to real-world exposure increased susceptibility to cardiac alternans after 12 weeks of exposure.85
In a study that compared 18 otherwise-healthy traditional cigarette smokers and 20 non-smokers, smoking menthol cigarettes increased tricuspid early diastolic peak flow velocity deceleration time and right ventricular isovolumic contraction time and decreased right ventricular early peak velocity compared to traditional cigarettes without menthol. However, both traditional and menthol cigarettes had negative acute effects on both left and right ventricular function. This potentially suggests that the menthol component in some traditional cigarettes may further negatively impact right ventricular excitation, contraction, and relaxation.86 However, the nicotine is hypothesized to be the main component in arrhythmogenic pathogenesis. Therefore, the mechanisms of arrhythmia are hypothesized to be similar for traditional combustible cigarettes and e-cigarettes. The role of the autonomic nervous system in arrhythmogenesis is well studied. The sympathetic and parasympathetic nervous system can incite heterogeneity in the function of ion channels that regulate excitation–contraction coupling. Modified autonomic nervous system has been shown to play a role in long QT and Brugada syndrome, and this can be displayed via HRV.87,88
5. Conclusion
This current review has summarized the concerning potential that 4th generation e-cigarettes have on inducing various arrhythmias within the heart. With the recent advent of 4th generation e-cigarettes, studies investigating the health effects are limited and ongoing. In fact, while numerous components of e-cigarettes are known to induce arrhythmias, the exact mechanism(s) remain undetermined. This knowledge gap is due in part to the variability within 4th generation e-cigarettes, with differences in flavourings, nicotine levels, and solvent ratios. This makes it increasingly difficult to conclusively determine health risks of e-cigarettes, as each e-cigarette is different and can produce distinct health effects. Future research should continue to focus on the long-term effects of 4th generation e-cigarettes, attempting to characterize the health effects of the multiple variables in e-cigarettes. Additionally, longitudinal studies investigating the cardiovascular health of e-cigarette users should be utilized to characterize physiological changes.
Unfortunately, e-cigarette popularity continues to grow exponentially, especially among adolescents. With limited research on health user data, the FDA has yet to place strict regulations on the distribution of e-cigarettes. Therefore, it becomes increasingly critical to research every aspect of these newly introduced e-cigarettes, to not only initiate FDA regulations but to begin to find new approaches in treating health issues caused by e-cigarette use.
Acknowledgement
All figures were created using Biorender.com.
Contributor Information
Carson A Jones, Dorothy M. Davis Heart and Lung Research Institute, Wexner Medical Center, The Ohio State University, 473 W 12th Avenue, Columbus, OH 43210, USA.
Michael J Wallace, Dorothy M. Davis Heart and Lung Research Institute, Wexner Medical Center, The Ohio State University, 473 W 12th Avenue, Columbus, OH 43210, USA.
Priya Bandaru, Dorothy M. Davis Heart and Lung Research Institute, Wexner Medical Center, The Ohio State University, 473 W 12th Avenue, Columbus, OH 43210, USA.
Emerson D Woodbury, Dorothy M. Davis Heart and Lung Research Institute, Wexner Medical Center, The Ohio State University, 473 W 12th Avenue, Columbus, OH 43210, USA.
Peter J Mohler, Dorothy M. Davis Heart and Lung Research Institute, Wexner Medical Center, The Ohio State University, 473 W 12th Avenue, Columbus, OH 43210, USA; Department of Internal Medicine, Wexner Medical Center, The Ohio State University, Columbus, OH, USA.
Loren E Wold, Dorothy M. Davis Heart and Lung Research Institute, Wexner Medical Center, The Ohio State University, 473 W 12th Avenue, Columbus, OH 43210, USA; Division of Cardiac Surgery, Department of Surgery, Wexner Medical Center, The Ohio State University, 473 W 12th Avenue, Room 603, Columbus, OH 43210, USA.
Funding
This work was supported by the National Institutes of Health [grants HL139348 and AG057046 to L.E.W., HL135754 to P.J.M.]; the American Heart Association [grant 20YVNR35490079 to P.J.M. and L.E.W.]; the Ohio State Frick Center for Heart Failure and Arrhythmia; and the Leducq Foundation [TNE FANTASY 19CV03 to P.J.M.].
References
- 1. Breland A, Soule E, Lopez A, Ramôa C, El-Hellani A, Eissenberg T. Electronic cigarettes: what are they and what do they do? Ann N Y Acad Sci 2017;1394:5–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dinardo P, Rome ES. Vaping: the new wave of nicotine addiction. Cleve Clin J Med 2019;86:789–798. [DOI] [PubMed] [Google Scholar]
- 3. Leventhal AM, Dai H, Barrington-Trimis JL, Tackett AP, Pedersen ER, Tran DD. Disposable e-cigarette use prevalence, correlates, and associations with previous tobacco product use in young adults. Nicotine Tob Res 2022;24:372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jones K, Salzman GA. The vaping epidemic in adolescents. Mo Med 2020;117:56–58. [PMC free article] [PubMed] [Google Scholar]
- 5. Neczypor EW, Mears MJ, Ghosh A, Sassano MF, Gumina RJ, Wold LE, Tarran R. E-cigarettes and cardiopulmonary health: review for clinicians. Circulation 2022;145:219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. American Lung Association. Overall tobacco trends. 2019. https://www.lung.org/research/trends-in-lung-disease/tobacco-trends-brief/overall-tobacco-trends (20 January 2023, date last accessed).
- 7. National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Prevention NCfCD. Preventing tobacco use among youth and young adults: a report of the Surgeon General. CDC2012. https://www.ncbi.nlm.nih.gov/books/NBK99237/ (26 January 2023, date last accessed). [PubMed]
- 8. Youth e-cigarette use remains serious public health concern amid COVID-19 pandemic CDC2021. https://www.cdc.gov/media/releases/2021/p0930-e-cigarette.html (7 February 2023, date last accessed).
- 9. [(6 October 2022, date last accessed).]. https://www.cdc.gov/media/releases/2022/p1007-e-cigarette-use.html#:∼:text=A%20study%20released%20today%20from, and%203.3%25%20of%20middle%20school More than 2.5 million youth reported e-cigarette use in 2022 CDC2022.
- 10. GBD 2019 Tobacco Collaborators . Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet (London, England) 2021;397:2337–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. [(29 July 2022, date last accessed).]. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/fast_facts/diseases-and-death.html Diseases and death CDC2022.
- 12. Nagawa CS, Ito Fukunaga M, Faro JM, Liu F, Anderson E, Kamberi A, Orvek EA, Davis M, Pbert L, Cutrona SL, Houston TK, Sadasivam RS. Characterizing pandemic-related changes in smoking over time in a cohort of current and former smokers. Nicotine Tob Res 2023;25:203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marques P, Piqueras L, Sanz MJ. An updated overview of e-cigarette impact on human health. Respir Res 2021;22:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Talih S, Salman R, El-Hage R, Karaoghlanian N, El-Hellani A, Saliba N, Shihadeh A. Effect of free-base and protonated nicotine on nicotine yield from electronic cigarettes with varying power and liquid vehicle. Sci Rep 2020;10:16263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shi H, Fan X, Horton A, Haller ST, Kennedy DJ, Schiefer IT, Dworkin L, Cooper CJ, Tian J. The effect of electronic-cigarette vaping on cardiac function and angiogenesis in mice. Sci Rep 2019;9:4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Navas-Acien A, Martinez-Morata I, Hilpert M, Rule A, Shimbo D, LoIacono NJ. Early cardiovascular risk in e-cigarette users: the potential role of metals. Curr Environ Health Rep 2020;7:353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gentzke AS, Wang TW, Cornelius M, Park-Lee E, Ren C, Sawdey MD, Cullen KA, Loretan C, Jamal A, Homa DM . Tobacco product use and associated factors among middle and high school students—National Youth Tobacco Survey, United States, 2021. MMWR Surveill Summ 2022;71:1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barrington-Trimis JL, Leventhal AM. Adolescents’ use of “pod mod” e-cigarettes—urgent concerns. N Engl J Med 2018;379:1099–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tarran R, Barr RG, Benowitz NL, Bhatnagar A, Chu HW, Dalton P, Doerschuk CM, Drummond MB, Gold DR, Goniewicz ML, Gross ER, Hansel NN, Hopke PK, Kloner RA, Mikheev VB, Neczypor EW, Pinkerton KE, Postow L, Rahman I, Samet JM, Salathe M, Stoney CM, Tsao PS, Widome R, Xia T, Xiao D, Wold LE. E-cigarettes and cardiopulmonary health. Function 2021;2:zqab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tsai M, Byun MK, Shin J, Crotty Alexander LE. Effects of e-cigarettes and vaping devices on cardiac and pulmonary physiology. J Physiol 2020;598:5039–5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. MacDonald A, Middlekauff HR. Electronic cigarettes and cardiovascular health: what do we know so far? Vasc Health Risk Manag 2019;15:159–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Voos N, Goniewicz ML, Eissenberg T. What is the nicotine delivery profile of electronic cigarettes? Expert Opin Drug Deliv 2019;16:1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol 2009;49:57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Benowitz NL, Burbank AD. Cardiovascular toxicity of nicotine: implications for electronic cigarette use. Trends Cardiovasc Med 2016;26:515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chaumont M, de Becker B, Zaher W, Culié A, Deprez G, Mélot C, Reyé F, Van Antwerpen P, Delporte C, Debbas N, Boudjeltia KZ, van de Borne P. Differential effects of e-cigarette on microvascular endothelial function, arterial stiffness and oxidative stress: a randomized crossover trial. Sci Rep 2018;8:10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Caporale A, Langham MC, Guo W, Johncola A, Chatterjee S, Wehrli FW. Acute effects of electronic cigarette aerosol inhalation on vascular function detected at quantitative MRI. Radiology 2019;293:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Prochaska JJ, Vogel EA, Benowitz N. Nicotine delivery and cigarette equivalents from vaping a JUULpod. Tob Control 2022;31:e88–e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. DeVito EE, Krishnan-Sarin S. E-cigarettes: impact of E-liquid components and device characteristics on nicotine exposure. Curr Neuropharmacol 2018;16:438–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kathuria H. Electronic cigarette use, misuse. And harm. Med Clin North Am 2022;106:1081–1092. [DOI] [PubMed] [Google Scholar]
- 30. American Academy of Pediatrics. Flavored e-cigarette and tobacco products. 2022. https://www.aap.org/en/advocacy/state-advocacy/flavored-e-cigarette-and-tobacco-products/#:∼:text=E%2DCigarettes%20have%20been%20marketed, cigarette%20smoking%20is%20not%20allowed. (22 February 2023, date last accessed).
- 31. Yingst JM, Veldheer S, Hammett E, Hrabovsky S, Foulds J. A method for classifying user-reported electronic cigarette liquid flavors. Nicotine Tob Res 2017;19:1381–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bonner E, Chang Y, Christie E, Colvin V, Cunningham B, Elson D, Ghetu C, Huizenga J, Hutton SJ, Kolluri SK, Maggio S, Moran I, Parker B, Rericha Y, Rivera BN, Samon S, Schwichtenberg T, Shankar P, Simonich MT, Wilson LB, Tanguay RL. The chemistry and toxicology of vaping. Pharmacol Ther 2021;225:107837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Khlystov A, Samburova V. Flavoring compounds dominate toxic aldehyde production during e-cigarette vaping. Environ Sci Technol 2016;50:13080–13085. [DOI] [PubMed] [Google Scholar]
- 34. Overbeek DL, Kass AP, Chiel LE, Boyer EW, Casey AMH. A review of toxic effects of electronic cigarettes/vaping in adolescents and young adults. Crit Rev Toxicol 2020;50:531–538. [DOI] [PubMed] [Google Scholar]
- 35. Brieger K, Schiavone S, Miller FJ, Krause K-H. Reactive oxygen species: from health to disease. Swiss Med Wkly 2012;142:w13659. [DOI] [PubMed] [Google Scholar]
- 36. Eltorai AE, Choi AR, Eltorai AS. Impact of electronic cigarettes on various organ systems. Respir Care 2019;64:328–336. [DOI] [PubMed] [Google Scholar]
- 37. Lerner CA, Sundar IK, Yao H, Gerloff J, Ossip DJ, McIntosh S, Robinson R, Rahman I. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One 2015;10:e0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lerner CA, Sundar IK, Watson RM, Elder A, Jones R, Done D, Kurtzman R, Ossip DJ, Robinson R, McIntosh S, Rahman I. Environmental health hazards of e-cigarettes and their components: oxidants and copper in e-cigarette aerosols. Environ Pollut 2015;198:100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Talih S, Salman R, Soule E, El-Hage R, Karam E, Karaoghlanian N, El-Hellani A, Saliba N, Shihadeh A. Electrical features, liquid composition and toxicant emissions from ‘pod-mod'-like disposable electronic cigarettes. Tob Control 2022;31:667–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Arrhythmias—types NHLBI NHLBI. 2022. https://www.nhlbi.nih.gov/health/arrhythmias/types (24 March 2022, date last accessed).
- 41. Wallace MJ, El Refaey M, Mesirca P, Hund TJ, Mangoni ME, Mohler PJ. Genetic complexity of sinoatrial node dysfunction. Front Genet 2021;12:654925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tse G. Mechanisms of cardiac arrhythmias. J Arrhythm 2016;32:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Frangogiannis NG. Cardiac fibrosis. Cardiovasc Res 2021;117:1450–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, Bonny A, Brauer M, Brodmann M, Cahill TJ, Carapetis J, Catapano AL, Chugh SS, Cooper LT, Coresh J, Criqui M, DeCleene N, Eagle KA, Emmons-Bell S, Feigin VL, Fernández-Solà J, Fowkes G, Gakidou E, Grundy SM, He FJ, Howard G, Hu F, Inker L, Karthikeyan G, Kassebaum N, Koroshetz W, Lavie C, Lloyd-Jones D, Lu HS, Mirijello A, Temesgen AM, Mokdad A, Moran AE, Muntner P, Narula J, Neal B, Ntsekhe M, de Oliveira G Moraes, Otto C, Owolabi M, Pratt M, Rajagopalan S, Reitsma M, Ribeiro ALP, Rigotti N, Rodgers A, Sable C, Shakil S, Sliwa-Hahnle K, Stark B, Sundström J, Timpel P, Tleyjeh IM, Valgimigli M, Vos T, Whelton PK, Yacoub M, Zuhlke L, Murray C, Fuster V; GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group . Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol 2020;76:2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Garcia PD, Gornbein JA, Middlekauff HR. Cardiovascular autonomic effects of electronic cigarette use: a systematic review. Clin Auton Res 2020;30:507–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Moheimani RS, Bhetraratana M, Yin F, Peters KM, Gornbein J, Araujo JA, Middlekauff HR. Increased cardiac sympathetic activity and oxidative stress in habitual electronic cigarette users: implications for cardiovascular risk. JAMA Cardiol 2017;2:278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tattersall MC, Hughey CM, Piasecki TM, Korcarz CE, Hansen KM, Ott NR, Sandbo N, Fiore MC, Baker TB, Stein JH. Cardiovascular and pulmonary responses to acute use of electronic nicotine delivery systems and combustible cigarettes in long-term users. Chest 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetière P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med 2005;352:1951–1958. [DOI] [PubMed] [Google Scholar]
- 49. Carll AP, Arab C, Salatini R, Miles MD, Nystoriak MA, Fulghum KL, Riggs DW, Shirk GA, Theis WS, Talebi N, Bhatnagar A, Conklin DJ. E-cigarettes and their lone constituents induce cardiac arrhythmia and conduction defects in mice. Nat Commun 2022;13:6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Perek S, Nussinovitch U, Cohen R, Gidron Y, Raz-Pasteur A. Ultra short heart rate variability predicts clinical outcomes in patients with a clinical presentation consistent with myocarditis: a derivation cohort analysis. J Clin Med 2022;12:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Singh N, Moneghetti KJ, Christle JW, Hadley D, Plews D, Froelicher V. Heart rate variability: an old metric with new meaning in the era of using mHealth technologies for health and exercise training guidance. Part one: physiology and methods. Arrhythm Electrophysiol Rev 2018;7:193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fox K, Ford I, Steg PG, Tendera M, Robertson M, Ferrari R. Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet 2008;372:817–821. [DOI] [PubMed] [Google Scholar]
- 53. Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health 2017;5:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Qiu H, Zhang H, Han DD, Derakhshandeh R, Wang X, Goyal N, Navabzadeh M, Rao P, Wilson EE, Mohammadi L, Olgin JE, Springer ML. Increased vulnerability to atrial and ventricular arrhythmias caused by different types of inhaled tobacco or marijuana products. Heart Rhythm 2023;20:76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Abouassali O, Chang M, Chidipi B, Martinez JL, Reiser M, Kanithi M, Soni R, McDonald TV, Herweg B, Saiz J, Calcul L, Noujaim SF. In vitro and in vivo cardiac toxicity of flavored electronic nicotine delivery systems. Am J Physiol 2021;320:H133–H143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. D'Alessandro A, Boeckelmann I, Hammwhöner M, Goette A. Nicotine, cigarette smoking and cardiac arrhythmia: an overview. Eur J Prev Cardiol 2012;19:297–305. [DOI] [PubMed] [Google Scholar]
- 57. Grant AO. Cardiac ion channels. Circ Arrhythm Electrophysiol 2009;2:185–194. [DOI] [PubMed] [Google Scholar]
- 58. Wang H, Shi H, Wang Z. Nicotine depresses the functions of multiple cardiac potassium channels. Life Sci 1999;65:PL143–PL149. [DOI] [PubMed] [Google Scholar]
- 59. Wang H, Shi H, Zhang L, Pourrier M, Yang B, Nattel S, Wang Z. Nicotine is a potent blocker of the cardiac A-type K(+) channels. Effects on cloned kv4.3 channels and native transient outward current. Circulation 2000;102:1165–1171. [DOI] [PubMed] [Google Scholar]
- 60. Demir V, Hidayet S, Turan Y, Ede H. Acute effects of electronic cigarette smoking on ventricular repolarization in adults. Afr Health Sci 2020;20:1793–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Feng Y, Caiping M, Li C, Can R, Feichao X, Li Z, Zhice X. Fetal and offspring arrhythmia following exposure to nicotine during pregnancy. J Appl Toxicol 2010;30:53–58. [DOI] [PubMed] [Google Scholar]
- 62. Zhu W, Yuan P, Shen Y, Wan R, Hong K. Association of smoking with the risk of incident atrial fibrillation: a meta-analysis of prospective studies. Int J Cardiol 2016;218:259–266. [DOI] [PubMed] [Google Scholar]
- 63. Shan H, Zhang Y, Lu Y, Zhang Y, Pan Z, Cai B, Wang N, Li X, Feng T, Hong Y, Yang B. Downregulation of miR-133 and miR-590 contributes to nicotine-induced atrial remodelling in canines. Cardiovasc Res 2009;83:465–472. [DOI] [PubMed] [Google Scholar]
- 64. Alexander LE C, Drummond CA, Hepokoski M, Mathew D, Moshensky A, Willeford A, Das S, Singh P, Yong Z, Lee JH, Vega K, Du A, Shin J, Javier C, Tian J, Brown JH, Breen EC. Chronic inhalation of e-cigarette vapor containing nicotine disrupts airway barrier function and induces systemic inflammation and multiorgan fibrosis in mice. Am J Physiol 2018;314:R834–R847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Podziemski P, Zeemering S, Kuklik P, van Hunnik A, Maesen B, Maessen J, Crijns HJ, Verheule S, Schotten U. Rotors detected by phase analysis of filtered, epicardial atrial fibrillation electrograms colocalize with regions of conduction block. Circ Arrhythm Electrophysiol 2018;11:e005858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nalivaiko E, De Pasquale CG, Blessing WW. Ventricular arrhythmias triggered by alerting stimuli in conscious rabbits pre-treated with dofetilide. Basic Res Cardiol 2004;99:142–151. [DOI] [PubMed] [Google Scholar]
- 67. Kurhanewicz N, McIntosh-Kastrinsky R, Tong H, Ledbetter A, Walsh L, Farraj A, Hazari M. TRPA1 Mediates changes in heart rate variability and cardiac mechanical function in mice exposed to acrolein. Toxicol Appl Pharmacol 2017;324:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hazari MS, Haykal-Coates N, Winsett DW, Krantz QT, King C, Costa DL, Farraj AK. TRPA1 and sympathetic activation contribute to increased risk of triggered cardiac arrhythmias in hypertensive rats exposed to diesel exhaust. Environ Health Perspect 2011;119:951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Khadka S, Awasthi M, Lamichhane RR, Ojha C, Mamudu HM, Lavie CJ, Daggubati R, Paul TK. The cardiovascular effects of electronic cigarettes. Curr Cardiol Rep 2021;23:40. [DOI] [PubMed] [Google Scholar]
- 70. Tanwar V, Adelstein JM, Wold LE. Double trouble: combined cardiovascular effects of particulate matter exposure and coronavirus disease 2019. Cardiovasc Res 2021;117:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fernández E, Ballbè M, Sureda X, Fu M, Saltó E, Martínez-Sánchez JM. Particulate matter from electronic cigarettes and conventional cigarettes: a systematic review and observational study. Curr Environ Health Rep 2015;2:423–429. [DOI] [PubMed] [Google Scholar]
- 72. Qasim H, Karim ZA, Rivera JO, Khasawneh FT, Alshbool FZ. Impact of electronic cigarettes on the cardiovascular system. J Am Heart Assoc 2017;6:e006353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Heeringa J, Kors JA, Hofman A, van Rooij FJ, Witteman JC. Cigarette smoking and risk of atrial fibrillation: the Rotterdam study. Am Heart J 2008;156:1163–1169. [DOI] [PubMed] [Google Scholar]
- 74. Zuo ML, Li CM, Deng Y, Bhattacharyya S, Shuai P, Tse HF, Siu C-W, Yin L-X. The impact of cigarette smoking in predicting stroke using CHADS(2) and CHA(2)DS(2)-VASc schemas. Neurol Sci 2021;42:159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chamberlain AM, Agarwal SK, Folsom AR, Duval S, Soliman EZ, Ambrose M, Eberly LE, Alonso A. Smoking and incidence of atrial fibrillation: results from the Atherosclerosis Risk In Communities (ARIC) study. Heart Rhythm 2011;8:1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zuo H, Nygård O, Vollset SE, Ueland PM, Ulvik A, Midttun Ø, Meyer K, Igland J, Sulo G, Tell GS. Smoking, plasma cotinine and risk of atrial fibrillation: the Hordaland Health Study. J Intern Med 2018;283:73–82. [DOI] [PubMed] [Google Scholar]
- 77. Groh CA, Vittinghoff E, Benjamin EJ, Dupuis J, Marcus GM. Childhood tobacco smoke exposure and risk of atrial fibrillation in adulthood. J Am Coll Cardiol 2019;74:1658–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cheng WH, Lo LW, Lin YJ, Chang SL, Hu YF, Hung Y, Chung F-P, Chang T-Y, Huang T-C, Yamada S, Salim S, Te ALD, Liao J-N, Tuan T-C, Chao T-F, Tsai T-Y, Liu S-H, Chen S-A. Cigarette smoking causes a worse long-term outcome in persistent atrial fibrillation following catheter ablation. J Cardiovasc Electrophysiol 2018;29:699–706. [DOI] [PubMed] [Google Scholar]
- 79. Goette A, Lendeckel U, Kuchenbecker A, Bukowska A, Peters B, Klein HU, Huth C, Rocken C. Cigarette smoking induces atrial fibrosis in humans via nicotine. Heart 2007;93:1056–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kors JA, Ritsema van Eck HJ, van Herpen G. The meaning of the Tp-Te interval and its diagnostic value. J Electrocardiol 2008;41:575–580. [DOI] [PubMed] [Google Scholar]
- 81. İlgenli TF, Tokatlı A, Akpınar O, Kılıçaslan F. The effects of cigarette smoking on the Tp-e interval, Tp-e/QT ratio and Tp-e/QTc ratio. Adv Clin Exp Med 2015;24:973–978. [DOI] [PubMed] [Google Scholar]
- 82. Goldenberg I, Moss AJ, McNitt S, Zareba W, Daubert JP, Hall WJ, Andrews ML. Cigarette smoking and the risk of supraventricular and ventricular tachyarrhythmias in high-risk cardiac patients with implantable cardioverter defibrillators. J Cardiovasc Electrophysiol 2006;17:931–936. [DOI] [PubMed] [Google Scholar]
- 83. Karakaya O, Barutcu I, Kaya D, Esen AM, Saglam M, Melek M, Onrat E, Turkmen M, Esen OB, Kaymaz C. Acute effect of cigarette smoking on heart rate variability. Angiology 2007;58:620–624. [DOI] [PubMed] [Google Scholar]
- 84. Qu Z, Weiss JN. Cardiac alternans: from bedside to bench and back. Circ Res 2023;132:127–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wang Z, Wang L, Tapa S, Pinkerton KE, Chen CY, Ripplinger CM. Exposure to secondhand smoke and arrhythmogenic cardiac alternans in a mouse model. Environ Health Perspect 2018;126:127001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ciftçi O, Calişkan M, Güllü H, Yildirir A, Müderrisoğlu H. Mentholated cigarette smoking induced alterations in left and right ventricular functions in chronic smokers. Anadolu Kardiyol Derg 2008;8:116–122. [PubMed] [Google Scholar]
- 87. Verrier RL, Antzelevitch C. Autonomic aspects of arrhythmogenesis: the enduring and the new. Curr Opin Cardiol 2004;19:2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Critselis E, Panagiotakos D. Impact of electronic cigarette use on cardiovascular health: current evidence, causal pathways, and public health implications. Angiology 2023. [DOI] [PubMed] [Google Scholar]