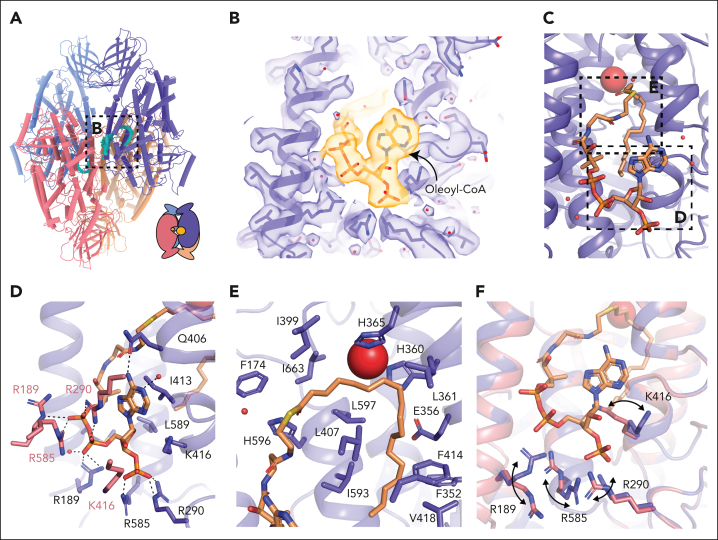
Figure 4.
Acyl-CoA binding site in the 12-LOX tetramer. (A) Model of a 12-LOX tetramer, with density in the catalytic site shown in cyan. The graphical representation is shown in the right corner. (B) Acyl-CoA model and the density. Density is shown as a transparent surface, and the model as sticks colored by the heteroatoms. (C) Model of acyl-CoA within the catalytic site of 12-LOX. (D-E) 12-LOX residues in contact with acyl-CoA (orange) are shown as sticks. (D) Interactions of the adenosine triphosphate group. (E) Interactions of the acyl tail. (F) Conformational changes in the residues in contact with acyl-CoA. Acyl-CoA--bound subunit is shown in purple and unbound is shown in pink. Iron atom is shown as a red sphere.