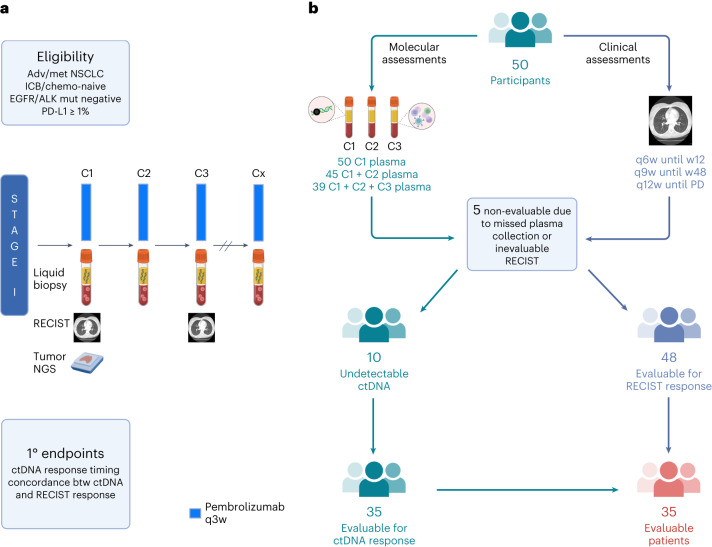
Fig. 1. BR.36 trial schema.
a, The first stage of the BR.36 trial enrolled patients with advanced/metastatic NSCLC who did not harbor clinically actionable genomic alterations in EGFR or ALK and had a PD-L1 expression level of ≥1%. Patients received pembrolizumab as per local standard of care, and RECIST radiographic response assessments were performed every 6 weeks until week 12 and at longer intervals thereafter (Methods). Serial liquid biopsies were collected before treatment administration on C1D1 (baseline), C2D1 (3 weeks) and C3D1 (6 weeks), followed by ctDNA molecular response assessments at these timepoints. The primary endpoints of the trial were to determine the optimal timepoint of ctDNA molecular response and validate the concordance of ctDNA molecular response with radiographic RECIST version 1.1 response. b, BR.36 reached its target enrollment of 50 patients; for each individual, serial radiographic assessments and liquid biopsy analyses were performed. C1D1 plasma was collected for all 50 patients; C1D1 and C2D1 plasma samples were collected for 45 patients; and plasma samples were collected for all three timepoints for 39 patients. Five patients were deemed not evaluable because of missed plasma collection or non-evaluable RECIST assessments. Of the 45 evaluable patients, 10 had undetectable ctDNA at all timepoints (no tumor-specific plasma variants detected), resulting in 35 patients with evaluable ctDNA and RECIST responses.