Fig. 2. CONSORT flow diagram.
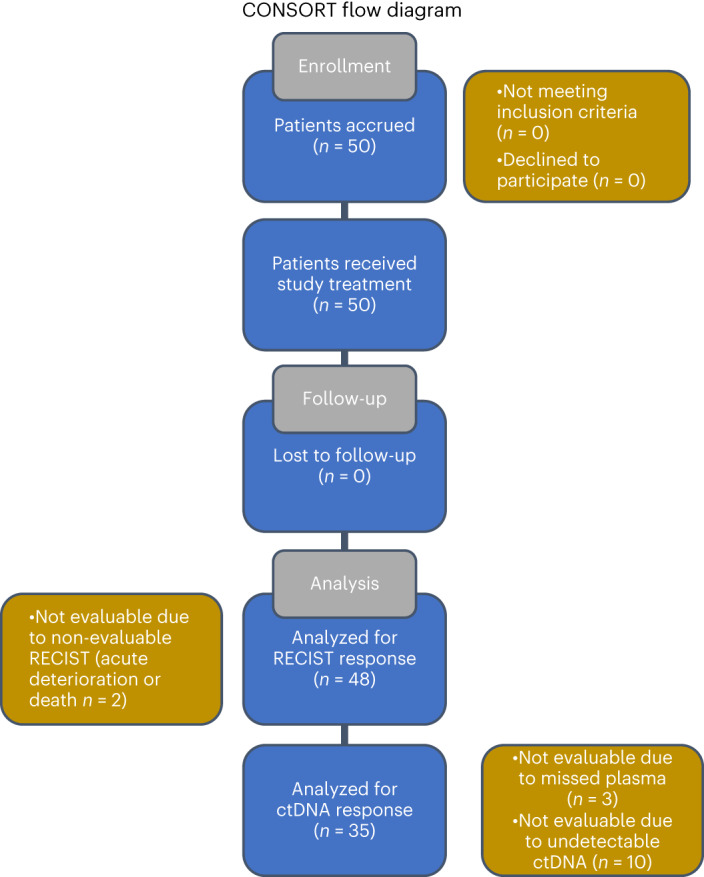
Of the 50 patients enrolled, two were non-evaluable for RECIST response because of symptomatic progression/acute deterioration/death during cycle 1 without imaging (BR360020 and BR360021). The remaining 96% (48/50) of patients in the BR.36 study were evaluable for radiographic response assessment, which supports the feasibility of CT restaging in this population. Of the 48 patients with evaluable radiographic responses, three were non-evaluable because of missed plasma collection (BR360014, BR360016 and BR360029) due to withdrawn consent, rapid disease progression/death and protocol violation, respectively. Of the 45 patients evaluable for both radiographic and ctDNA responses, 22.2% (10/45) had undetectable ctDNA, which is consistent with previously reported ctDNA undetectable rate in patients with metastatic NSCLC (Anagnostou et al.6) and was within the CI of the undetectable ctDNA rate that we factored in the sample size calculations for the BR.36 stage 1 cohort. adv, advanced; btw, between; met, metastatic; mut, mutation; q6w, once every 6 weeks; q9w, once every 9 weeks; q12w, once every 12 weeks; w, week.
