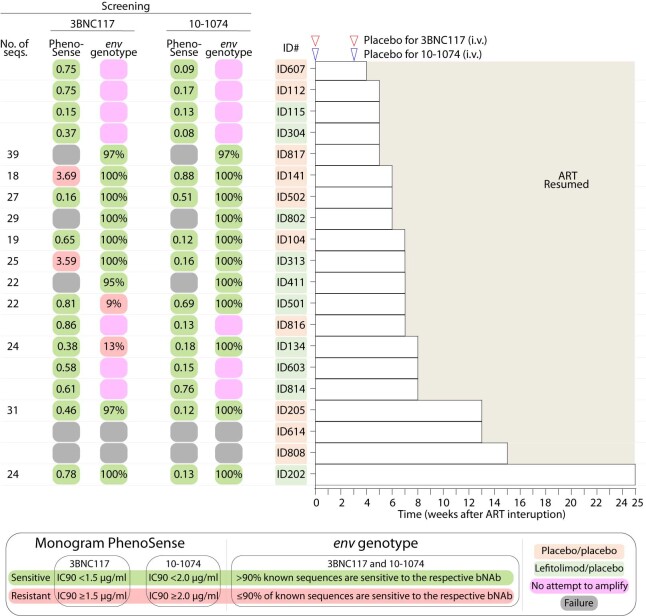
Extended Data Fig. 4. bNAb sensitivity at screening for the placebo/placebo and lefitolimod/placebo groups.
bNAb sensitivity was primarily analyzed using the PhenoSense Monoclonal Antibody Assay with predefined IC90 thresholds for 3BNC117 ( < 1.5 µg/mL) and 10-1074 ( < 2.0 µg/mL) and maximum percentage inhibition (MPI) ≥ 98%. In seven participants, the PhenoSense Assay failed, and we secondarily used proviral HIV-1 envelope (env) sequences on a genotypic prediction algorithm with predefined thresholds of ≥90% known sequences are sensitive. Two participants (ID141 and ID313) were enrolled based upon the genotypically analysis, but post-hoc phenotypic data showed IC90 values of 3.69 and 3,59 µg/mL for 3BNC117, respectively. In another two participants (ID614 and ID808), both the PhenoSense Assay and env sequencing initially failed, and we tertiary-included (see Methods) these individuals based on the assumption that both assays failed due to a very small reservoir size. Empty red and blue triangles indicate placebo for 3BNC117 and 10-1074 infusions, respectively. Light grey shaded areas indicate time on ART, while white shaded boxes indicate still interrupting ART during the 25 weeks of ATI. Dark grey boxes are assay failures and pink boxes are no attempt to amplify. ART, antiretroviral therapy; ATI, ART interruption; env; HIV-1 envelope; IC90, concentration of bNAb required for 90% inhibition; i.v., intravenous.