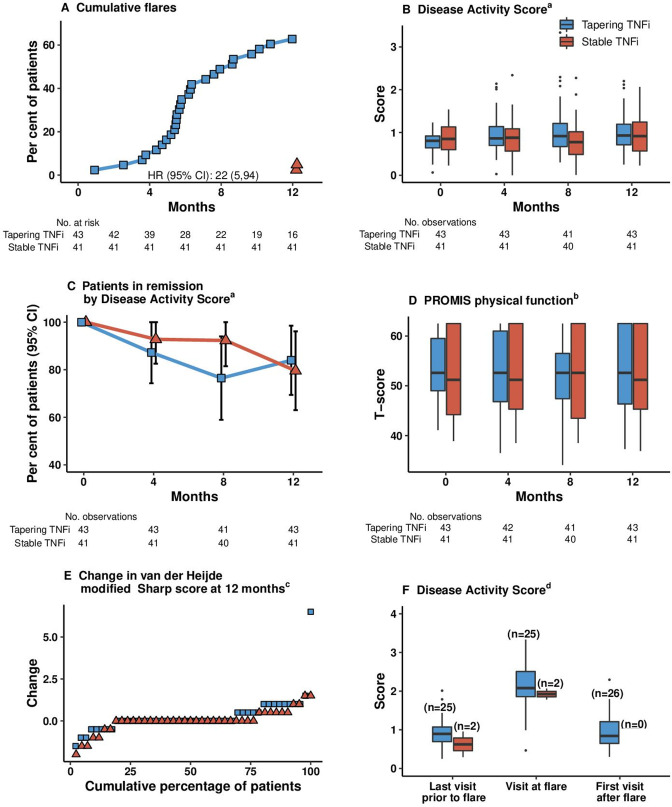
Figure 3.
Secondary endpoints. Analysed in the primary analysis population, defined as all randomised patients meeting the study entry criteria and with no protocol deviations affecting the treatment efficacy. Patients were followed up for a median (IQR) of 364 days (358–378 days) in the tapered group and 366 days (357–378 days) in the stable group. Variables are displayed based on clinical relevance. Boxes mark first and third quartiles, the band inside the box is the second quartile (the median), while the whiskers indicate the highest and lowest values within 1.5 × the IQR. Dots denote individual patients (outliers).a Disease Activity Score (DAS, range 0–10) includes a 44 swollen joint count assessment of tender joints by the Ritchie Articular Index, the erythrocyte sedimentation rate (ESR) and patient’s global assessment of disease activity on a VAS 0–100 mm. Remission is defined as any value below 1.6, low disease activity 1.6–2.4, moderate disease activity >2.4–3.7 and high disease activity >3.7, thus higher scores indicating more disease activity. bPROMIS assesses the ability to perform basic and instrumental activities of daily living. The total score is translated into a T score with a mean (SD) of 50 (10). A score of 50 equals the average for the general US population. cThe van der Heijde–modified Sharp scoring method assesses erosions in 16 joints of each hand and 6 joints of each foot, and the erosions are given a score of 1 to 5. Joint space narrowing is assessed in 15 joints for each hand and 6 joints for each foot. This gives scores for erosions on a scale of 0–280 and joint space narrowing on a scale of 0–168, thus the total van der Heijde–modified Sharp score ranges from 0 to 448, with higher scores indicating greater joint damage. A good radiographic outcome is commonly defined as no progression. dDisease Activity Score at the visit before a flare occurred, at the flare visit, and at visits after flare in the half-dose arm in those with all components available to calculate DAS. A flare could be recorded both at regular visits and at additional visits. PROMIS, Patient-Reported Outcomes Measurement Information 20-item Short Form Physical Function; TNFi, tumour necrosis factor inhibitor; VAS Visual Analogue Scale.