Abstract
Biological nitrogen fixation (BNF), the conversion of N2 into bioavailable nitrogen (N), is the main process for replenishing N loss in the biosphere. However, BNF in groundwater systems remains poorly understood. In this study, we examined the activity, abundance, and community composition of diazotrophs in groundwater in the Hetao Plain of Inner Mongolia using 15N tracing methods, reverse transcription qPCR (RT-qPCR), and metagenomic/metatranscriptomic analyses. 15N2 tracing incubation of near in situ groundwater (9.5–585.4 nmol N L−1 h−1) and N2-fixer enrichment and isolates (13.2–1728.4 nmol N g−1 h−1, as directly verified by single-cell resonance Raman spectroscopy), suggested that BNF is a non-negligible source of N in groundwater in this region. The expression of nifH genes ranged from 3.4 × 103 to 1.2 × 106 copies L−1 and was tightly correlated with dissolved oxygen (DO), Fe(II), and NH4+. Diazotrophs in groundwater were chiefly aerobes or facultative anaerobes, dominated by Stutzerimonas, Pseudomonas, Paraburkholderia, Klebsiella, Rhodopseudomonas, Azoarcus, and additional uncultured populations. Active diazotrophs, which prefer reducing conditions, were more metabolically diverse and potentially associated with nitrification, sulfur/arsenic mobilization, Fe(II) transport, and CH4 oxidation. Our results highlight the importance of diazotrophs in subsurface geochemical cycles.

Subject terms: Microbial ecology, Biogeochemistry
Introduction
Biological nitrogen fixation (BNF) is a process by which microorganisms convert N2 into bioavailable nitrogen (N); it contributes 50% of the annual total available N in the biosphere, sustaining active nitrification and N loss processes [1]. BNF is mediated by three types of nitrogenases with similar structure and function, but different active site metal cofactors: Nif (molybdenum-iron [Fe]), Vnf (vanadium-iron), and Anf (Fe-Fe) nitrogenases [2]. The nifH gene encoding the nitrogenase Fe protein is used as a biomarker for detecting diazotrophic abundance and community composition in aquatic and terrestrial environments [3, 4]. Cyanobacteria are the most important diazotrophs in marine environments; these include Trichodesmium, Richelia, and three groups of unicellular Cyanobacteria (UCYN-A, UCYN-B, and UCYN-C) [5–7]. In terrestrial ecosystems, diazotrophs mainly belong to Pseudomonadota, Bacillota, and Actinomycetota [8, 9].
N2 fixation has also been detected in diverse habitats such as oceans, estuaries, lakes, and forests through 15N2 tracing (direct) and acetylene reduction assays (indirect) [4, 10, 11]. Oxygen levels [12, 13], N [5, 14], phosphorus (P) [15, 16], carbon (C) [3, 10], and trace metal concentrations [9, 17] are commonly implicated as the main influencing factors of N2 fixation, which vary significantly across different habitats. In addition, diazotrophs may use Fe, S, and C as terminal electron acceptors [18], and the coupling of Fe(III)/sulfate reduction and N2 fixation may be widespread in marine sediments and soils [3, 19–22]. Thus, diazotrophs appear to be habitat-dependent stable sources of N in both oligotrophic tailings and hypereutrophic estuaries [10, 23], and exploring their distribution, activity, and controlling factors is crucial for understanding the environmental effects and interconnected biogeochemical networks of BNF.
As the largest distributed freshwater reservoir worldwide, groundwater plays a central part in the hydrological cycle through discharge and recharge dynamics [24]. Groundwater systems are light-deprived and generally characterized by long water residence times and geochemical heterogeneity [25]. Anthropogenic activities, such as agricultural irrigation, livestock breeding, and domestic wastewater discharge increase the input of organic C, N, and other chemicals into groundwater systems, which can accelerate N cycling [26–29]. The mineralization of organic N is the main source of NH4+ in groundwater [30]. Studies of the groundwater N cycle have mainly focused on tracing N and its transformation to NH4+, NO2−, and NO3− [31, 32]. Researchers have also investigated the distribution of microorganisms related to N cycling in groundwater at varying oxygen levels through metagenomic and metatranscriptomic sequencing [1]. Denitrification, anammox, and Fe(III) reduction coupled with ammonium oxidation (Feammox) appear to be the dominant pathways of N2 production in aquifers [28, 29]. The vadose zone connects surface soil and groundwater and probably provides considerable N2 to groundwater via denitrification or anammox [33–35]. Active N2 generation has also been observed in anoxic groundwater [1, 36]. Together, these findings indicate excess N2 and reducing substances in groundwater [32]. However, still is known about N2 fixation in groundwater. Filling this N2 fixation knowledge gap would provide a more comprehensive understanding of the N cycle in groundwater systems.
N2 fixation rate measurements have rarely been conducted in field groundwater systems. GeoChip analysis and high-throughput sequencing detected nifH genes in groundwater, suggesting that BNF is an essential source of groundwater N [32, 37, 38]. Recently, many new nitrogenases and nitrogenase homologs have been found in groundwater-associated Elusimicrobia genomes using metagenomic methods [39]. However, none of these studies has analyzed the N2 fixation and diversity of diazotrophs in detail. Current knowledge of N2 fixation in groundwater systems is based primarily on the functional prediction of 16S rRNA genes or metagenomic sequencing. N2-fixing strains affiliated with Mesorhizobium and Azospira have been isolated from groundwater [40, 41]; however, their N2 fixation activity has yet to be verified. Thus, there may be many active N2-fixing microorganisms in aquifers that are involved in or influence other biogeochemical processes. Nevertheless, convincing evidence for N2 fixation activity in groundwater is still limited.
In this study, we investigated the abundance, diversity, and community composition of native/active N2-fixing bacteria in groundwater using reverse transcription PCR (RT-qPCR) and high-throughput sequencing by targeting nifH genes and transcripts. We also explored the geochemical factors controlling diazotrophs and the relationship between N2 fixation and other biogeochemical processes based on metagenomic and metatranscriptomic analyses, and verified diazotroph activity in groundwater using 15N2 tracing, single-cell resonance Raman (RR) spectroscopy with 15N stable isotope probing (SIP), and acetylene reduction assays.
Materials and methods
Sample collection and geochemical analysis
We collected groundwater samples in the Hetao Plain, Inner Mongolia, China (40°40–41°50’N, 106°45–107°90’E) (Fig. S1). The study region has an arid/semiarid climate, and its aquifers feature strong geochemical–microbial interactions involving N, C, S, arsenic (As), and Fe. Groundwater generally flows from oxidizing to reducing areas, potentially creating a redox gradient that supports multiple biochemical cycles [42]. The study area was described in detail in our previous study [32].
Groundwater samples (G1–G30) were collected directly from tube wells using Tygon tubing and a vacuum pump at depths of 15–100 m in Hangjinhouqi County, in the western part of the Hetao Plain. Before sampling, the wells were pumped slowly and uniformly (approximately 0.5 L/min) [43] for at least 20 min to obtain stable oxidation–reduction potential (ORP), temperature (T), and dissolved oxygen (DO). Water samples for determining N2 fixation rates were subsampled into 1-L high-density polyethylene (HDPE) bottles (Nalgene, Rochester, NY, USA) that had been acid-washed and groundwater-rinsed three times; the bottled samples were kept in the dark in black plastic bags during collection and incubation. Groundwater biomass was collected by filtering approximately 10 L of water with 0.22 μm filters (Millipore, Burlington, MA, USA) for DNA extraction and 2500 L of water with tangential flow filtration using a Prep/Scale spiral-wound cartridge with a 0.22 μm molecular weight cutoff (Millipore) for RNA extraction. The standard filters were packaged into 50 mL sterile tubes (NEST Biotechnology, Wuxi, China) and then frozen for DNA extraction, qPCR, and nifH gene high-throughput sequencing. The cell suspension concentrated by tangential flow filtration was packed into 500 mL sterile nuclease-free HDPE bottles (Nalgene) and stored on dry ice. RNA was extracted for RT-qPCR and metatranscriptomic sequencing on the same day in the local Centers for Disease Control and Prevention (CDC) laboratory. The pipettes and centrifuge tubes used in the extraction were treated with 0.1% diethyl pyrocarbonate (Aladdin, Shanghai, China) to prevent RNase contamination. Groundwater samples for measuring dissolved organic carbon (DOC) were collected in 60 mL brown HDPE bottles (Nalgene) that were quickly acidified with 1% v/v HNO3, sealed; samples were then stored on dry ice and determined within 3 days using a TOC analyzer (TOC-L; CPH, Shimadzu, Japan). Geochemical characteristics including T, pH, DO, ORP, and the concentrations of SO42−, NO3−, NO2−, NH4+, Fe(II), and total iron (FeTot), in water samples were measured in the field with a Hach spectrophotometers (HachDR890 and Hach40D, Loveland, CA, USA), according to the manufacturer’s instructions. Arsenic species were separated as described in our previous study [44].
Nucleic acid extraction, cDNA synthesis, and qPCR
DNA and RNA were extracted from all samples using the FastDNA SPIN (MP Biomedicals, Santa Ana, CA, USA) and RNeasy PowerSoil Total RNA (Qiagen, Hilden, German) kits, respectively, following the manufacturer’s protocols. DNA from N2-fixing strains isolated from groundwater was extracted using the MiniBEST Bacteria Genomic DNA Extraction Kit v3.0 (TaKaRa Bio, Shiga, Japan). The quality and quantity of DNA/RNA were examined using a NanoDrop-2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and agarose (1%) (Biowest, Riverside, MO, USA) gel electrophoresis. The residual DNA in RNA was eliminated using Recombinant DNase I (TaKaRa Bio), and then cDNA was synthesized using a PrimeScript II First Strand cDNA Synthesis Kit (TaKaRa Bio). All DNA, cDNA, and RNA samples were stored at −80 °C for quantitative and sequencing analysis.
The abundance of nifH and 16S rRNA genes (V4 region) (DNA- and RNA-based) in groundwater samples was quantified (Applied Biosystems, Waltham, MA, USA); the primers and amplification conditions are described in Table S2. qPCR was performed in triplicate. Plasmids were extracted from Escherichia coli DH5α hosts using the MiniBEST Plasmid Purification Kit (TaKaRa Bio). Standard curves were constructed and quantified by 10-fold dilutions of the plasmids. The correlation coefficients (R2) for all the genes exceeded 0.95, and amplification efficiency in this study ranged from 92% to 97%.
PCR amplification, sequencing, and data analysis
The nifH genes in groundwater samples were amplified by PCR (T100, Bio-Rad, Hercules, CA, USA) with universal primers PolF (with an added unique 8-bp barcode sequence for sample sorting) and PolR [45]. The 16S rRNA genes in groundwater samples and N2-fixer enrichments were amplified using primers 515 F and 806 R, targeting the 16S rRNA gene V4 region of microorganisms. The PCR reaction mixture and programs are described in detail in Table S2. The full-length 16S rRNA genes were amplified by PCR using primers 27 F/1492 R. The PCR products were detected using gel electrophoresis (1%) and purified using a MiniBEST agarose gel DNA extraction kit (TaKaRa, Bio).
The PCR products of the 16S rRNA genes were sent to Sangon Biotech (Shanghai, China) for sequencing using an Applied Biosystems 3730 DNA Analyzer and the sequences were deposited in the National Center for Biotechnology Information (NCBI) GenBank (accession nos: OQ561323-OQ561328). The nifH amplicons were paired-end sequenced (2 × 250 bp) on a NovaSeq platform (Illumina, San Diego, CA, USA) according to the standard protocol of Aikangjian (Wuhan, China). The 16S rRNA amplicons of groundwater and N2-fixer enrichments were paired-end sequenced (2 × 250 bp) on a NovaSeq platform by Magigene Technology (Guangzhou, China).
The raw sequencing data were processed using a Galaxy-based pipeline in Ye Deng’s laboratory at the Research Center for Eco-Environmental Sciences, Chinese Academy of Science, China (http://mem.rcees.ac.cn:8080). Detailed processing descriptions are shown in Supporting Information S1. The raw sequencing data of nifH genes and 16S rRNA genes were deposited in the NCBI database under accession nos PRJNA937386, PRJNA996345, and PRJNA996367. A representative groundwater flow field (G1, G2, G27, and G30) was selected from the recharge areas (near a mountain, hypoxic conditions) to the discharge areas (anaerobic conditions) for metagenomic and metatranscriptomic analyses. Groundwater total DNA and RNA were sent to Beijing Novogene Bioinformatics Technology for metagenomic and metatranscriptomic sequencing using a HiSeq platform (Illumina). The raw sequences were deposited in the NCBI database (accession nos: PRJNA882225 and PRJNA884812). The raw sequences were trimmed, assembled, filtered for quality control, and annotated. Binning was conducted using MetaWRAP.26 v1.3 pipeline with the default parameters [46]. Further data processing is detailed in the Supporting Information S1 and our previous study [47].
Enrichment and isolation of N2-fixer strains
Groundwater samples G1, G27, G28, G29, and G30, which were collected along a typical groundwater flow path [48], were used to enrich diazotrophs. First, a piece of filtered membrane was cut and transferred to 100 mL of sterilized enrichment medium (N-free Burk’s culture medium) containing (per liter) 20 g mannitol (BioFroxx; neoFroxx, Einhausen, Germany), 0.2 g KH2PO4, 0.8 g·K2HPO4, 0.2 g MgSO4·7H2O, 0.1 g CaSO4·2H2O (SCR), and trace amounts of Na2MoO4·2H2O (Sigma-Aldrich, St. Louis, MO, USA) and FeCl3 (Sigma-Aldrich). The initial pH of the medium was adjusted to 7.0 and then it was incubated at 30 °C and 120 rpm under anaerobic conditions (purged with N2 for 40 min). After 5 days, 10 mL of the culture was removed and transferred into 100 mL of fresh Burk’s medium. The culture process was repeated three times, and N2-fixer enrichments G1, G27, G28, G29, and G30 were cultured at 30 °C to determine the N2 fixation activity. To isolate N2-fixing strains, the enrichments were serially diluted 10-fold, and 50 μL cultures with 10−6 dilution were plated on LB solid medium (pH 7.0) containing 10 g tryptone (Oxoid, Basingstoke, UK), 5 g yeast extract (Oxoid), 10 g NaCl (SCR), and 15 g agar L−1 (BioFroxx), and deionized water. All plates were incubated at 30°C for 5 days, and then single colonies were picked and subcultured on Burk’s medium three times in succession. The positive strains were preserved in 20% glycerol (SCR) at −80 °C for further experiments.
Characterization of N2 fixation
Immediately after water sampling, N2 fixation rates were measured at sampling stations in triplicate using the 15N-pulse tracing method following the incubation workflow (Fig. S2). Briefly, 4 mL 15N2 (Cambridge Isotope Laboratories, > 98 atom% 15N2, Lot #: I-24583/AR0664758) was injected into each HDPE bottle for incubation and then shaken gently for 15 min to dissolve tracer bubbles into the water samples [49, 50]. Then, the bottles were incubated at near in situ temperature ( ± 2 °C) in the dark. After approximately 16 h and 106 h, the incubation was stopped by filtration through precombusted (450 °C for 4 h) 25 mm glass fiber filters (GF75; Whatman, Maidstone, UK) to obtain particulate N (PN) and the culture for total dissolved N (TDN) was filtered through 0.2 µm syringe filters. Time zero (T0) control samples were processed as described above immediately after adding 15N2 tracer [51]. PN and TDN concentrations were determined using the wet chemical oxidation method [52]. Then, the oxidized NO3− was measured using a chemiluminescence method [30, 53]. The δ15N of PN and TDN were analyzed using a GasBench-II (Thermo Fisher Scientific) connected to an isotope ratio mass spectrometer (Delta V Advantage; Thermo Fisher Scientific). We have tested the used 15N2 gas bottle in this study for contamination with bioavailable non-N2 15N in previous studies [54, 55] as mentioned in Dabundo et al. [56]. Briefly, triplicate 2 mL 15N2 gas and 10 mL surface seawater (frome South China and 0.2 μm filtration) were injected into 20 mL headspace vials, sealed with a septum stopper, and then shaken overnight. The δ15N of TDN was measured and compared with the δ15N of natural surface seawater samples (frome South China and 0.2 μm filtration). Values of δ15N TDN of the blank seawater and test seawater groups were 4.3 ± 0.2 ‰ and 4.2 ± 0.3 ‰, respectively, suggesting no contamination of the 15N2 gas. Meanwhile, values of δ15N-NH4+ and δ15N-NOx− in the control and experimental groups (T0) in different groundwater samples are shown in Table S3. In addition, the nitrogenase activity of the in situ groundwater was also characterized by the acetylene-reduction assays [57]. Detailed descriptions of the 15N analysis method, N2 fixation rate calculation, and acetylene-reduction assays are provided in Supporting Information S2, S3.
The N2-fixer enrichment and isolate activity were detected using the 15N2 assimilation method [11]. In brief, 1 mL N2-fixer enrichments and bacterial suspensions (containing approximately 107 CFU) were inoculated in 60 mL gas-tight serum bottles containing 30 mL fresh N-free Burk’s medium (sterile and purged with argon for 30 min). Then, 3 mL of headspace air was extracted and replaced with 3 mL 15N2 gas (99 atom%, Lot no.210610; Reer Technology, Shanghai, China) using a gas-tight syringe. The bottles were placed upside down and incubated in a shaker at a constant temperature of 30 °C and 120 rpm for 48 h. After incubation, the N2-fixer enrichment and isolate suspensions were centrifuged at 8000 × g for 20 min at 4 °C for biomass collection. The samples were dried after being frozen overnight at −80 °C and then placed in a vacuum freeze dryer (Biocool AB, Stockholm, Sweden) for 2 days. Dried samples (0.03–0.05 mg) and urea (0.15–0.20 mg) were placed in a tin capsule for 15N-enrichment analysis using an Isolink-Delta V elemental analyzer (Thermo Fisher Scientific). The abundance of 15N in the N2-fixer enrichments and isolates (15N%) was calculated as follows [58]:
Where At15N% is the percentage of 15N in total N (measured by IRMS), Murea and Mbacteria are the weights of urea and sample in the tin capsule, and the 15N% in urea (15N%urec) is 0.367% (natural abundance). The total N contents in urea (Nurea) and bacteria (Nbacteria) were 46.7% and 9.8%, respectively.
To demonstrate N2-fixer activity more strongly, we used an acetylene-reduction assay, single-cell RR spectroscopy, and 15N2 SIP [58, 59]. The cultivation methods and procedures are described in Supporting Information S3.
Statistical analysis
Principal component analysis (PCA) was performed to evaluate differences in geochemical parameters based on Bray‒Curtis dissimilarities. The influence of geochemical factors on diazotrophic community composition was determined using R v4.1.2 for Pearson correlation and Canoco5 [canonical correlation analysis (CCA)] software. Nonmetric dimensional scaling was used to evaluate differences in diazotrophic community composition based on the Bray‒Curtis dissimilarity matrix of operational taxonomic units using the R vegan package. Pearson correlations between gene abundances and geochemical characteristics in groundwater were computed using R v4.1.2 and Chiplot (https://www.chiplot.online/tvbot.html). Co-occurrence and coexpression networks of microbial interactions in the groundwater dataset were calculated using the R igraph package and visualized in Gephi v0.9.7 [60, 61]. Data were plotted and fitted using GraphPad Prism v9 (GraphPad Software, San Diego, CA, USA). Phylogenetic trees of all strains were constructed using MEGA X.
Results
Geochemical parameters of groundwater samples
The geochemical parameters of groundwater samples showed that DO concentrations ranged from 0.4 to 2.2 mg L−1, and varied consistently with the ORP values (−168.8 to 88 mv) (Fig. S3 and Table S1). PCA divided the groundwater samples into two groups: oxidizing and reducing areas (Fig. S4A). Relatively high concentrations of electron donors, such as As(III), Fe(II), NH4+, and DOC, accumulated in the reducing area (Fig. S3). The NO3− concentrations were higher in the oxidizing area than in the reducing area (p < 0.01) (Fig. S4B). SO42− concentrations were also higher in the oxidizing area and were negatively correlated with As(III) (p < 0.05) and Fe(II) (p < 0.01) (Figs. S3C, S4B). Groundwater samples were either neutral or weakly alkaline (pH 7.4–9.4). A portion of the groundwater temperatures ranged from 11.2 to 17.7 °C and increased with ORP. Total N (TN) concentrations fluctuated between 2 and 8 mg L−1 and were negatively correlated with SO42− (Fig. S4B). These characteristics suggested a significant difference in geochemical conditions between oxidizing and reducing groundwater samples.
Quantification of nifH genes in groundwater samples
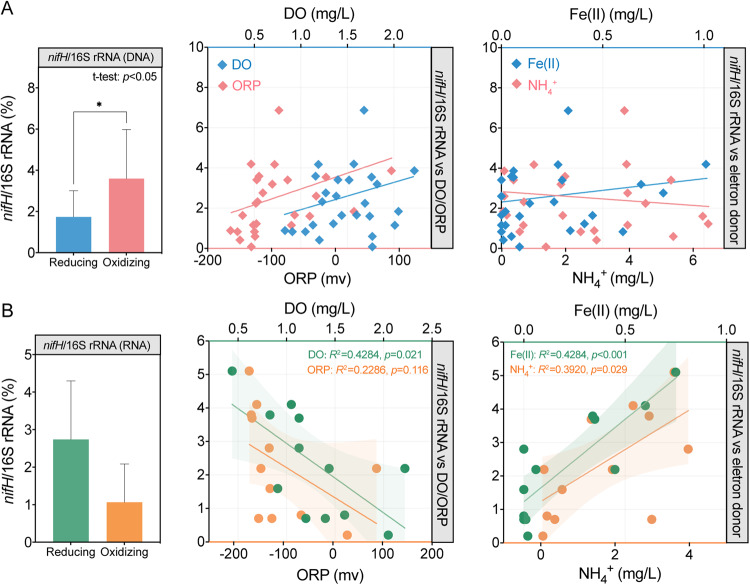
We assessed the abundances of the nifH genes in groundwater samples (Fig. 1, Table S4, and Table S5). At the gene level, the average abundance of nifH was 2.9 × 107 copies L−1 in the oxidizing area and 2.3 × 107 copies L−1 in the reducing area. The relative abundance of nifH (nifH/16S rRNA) genes was significantly elevated in the oxidizing area (p < 0.05) (Fig. 1A). Pearson correlation and linear regression analyses indicated that nifH/16S rRNA genes were positively correlated with DO (p < 0.05, r = 0.46). By contrast, the average abundance of nifH transcripts in the oxidizing area (1.2 × 105 copies L−1) was lower than that in the reducing area (3.2 × 105 copies L−1). The distribution of nifH/16S rRNA genes was consistent with the trend in transcript abundance (Fig. 1B). Correlation analysis showed that nifH/16S rRNA genes were negatively correlated with DO (p < 0.05, r = –0.65) at the transcriptional level, and Fe(II) was positively correlated with the expression of nifH genes (p < 0.001, r = 0.84) (Fig. S4B). PCA also showed differences in nifH/16S rRNA genes and their expressions under redox conditions (Fig. S4A).
Fig. 1. Distribution of nifH gene abundances and their response to environment variables.
Comparison of (A) DNA-based and (B) RNA-based nifH/16S rRNA genes (%) between the reducing area and oxidizing area and linear correlations between nifH/16S rRNA genes and DO, ORP, Fe(II), and NH4+ concentrations. The symbol * indicates significant differences between the reducing area and oxidizing area at p < 0.05. Solid lines represent linear regression modules. The shaded area represents the 95% confidence interval.
Diversity, community composition, and influencing factors of diazotrophs
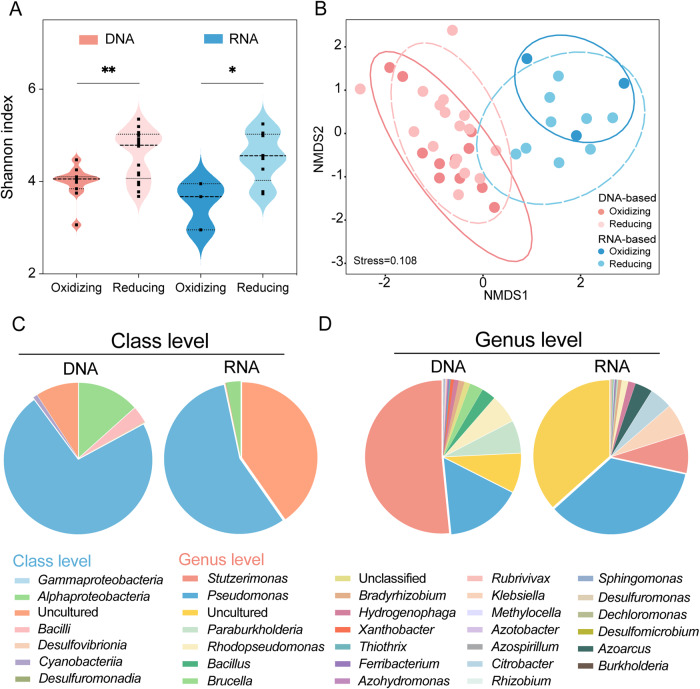
Both DNA- and RNA-based nifH genes were sequenced to investigate the community composition of N2-fixing bacteria in groundwater (Fig. 2). The Shannon indexes of DNA- and RNA-based diazotrophic communities were higher in the reducing area than in the oxidizing area (p < 0.05 and p < 0.01, respectively) (Fig. 2A), suggesting that redox conditions influence the diversity of groundwater-associated diazotrophs. However, gene and transcription levels influenced the community composition of diazotrophs more than redox conditions (Fig. 2B and Table S6). Phylum Pseudomonadota dominated N2-fixers in groundwater. At the class level, Gammaproteobacteria, Alphaproteobacteria, and uncultured populations were the most abundant diazotrophs in nifH amplicons (Fig. 2C), metagenomes, and metatranscriptomes (Fig. S5). The amplicon and genome results consistently showed that Cyanobacteria are native diazotrophs in groundwater (Figs. 2C and S5A). Further investigation of nifH amplicons at the genus level indicated that Stutzerimonas, Pseudomonas, and uncultured microbial populations were abundant in all groundwater samples, whereas Klebsiella was significantly enriched in the RNA-based analysis (Fig. 2D). The higher contribution of the uncultured microbial populations at the transcriptional level suggested that there are many unknown groups of active N2-fixing microbes in the underground environment.
Fig. 2. Diversity and community composition of native and active diazotrophs in groundwater samples.
A Comparison of the diversity index between oxidizing and reducing areas based on DNA and RNA levels (* and ** represent p < 0.05 and 0.01, respectively). B Nonmetric multidimensional scaling (NMDS) based on the Bray‒Curtis distance of diazotrophic communities. The relative abundances at the (C) class and (D) genus levels are based on the DNA/RNA level nifH gene amplicon sequencing.
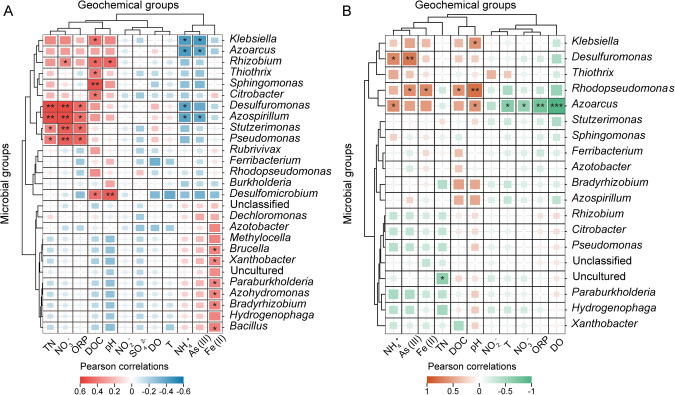
Environment–microbe interactions between N2 fixers and geochemical factors were characterized by CCA and Pearson correlation analyses (Figs. S6 and 3). CCA revealed that ORP, DOC, and NO2− (p < 0.05) were significantly correlated with the native diazotrophic communities, whereas active diazotrophs were potentially influenced by TN and NH4+ concentrations (p < 0.05) (Fig. S6). Pearson correlation showed that some diazotrophic populations were positively or negatively correlated with Fe(II), As(III), and DOC (Fig. 3). The abundances of aerobic or facultative anaerobic diazotrophs such as Azospirillum, Rhizobium, Stutzerimonas, Pseudomonas, and Desulfuromonas were positively correlated with ORP, NO3−, and TN (Fig. 3A). However, Desulfuromonas, Rhodopseudomonas, and Azoarcus transcript abundances were positively associated with Fe(II), As(III), and NH4+ (Fig. 3B), suggesting that N2 fixation might tightly interconnect with N, Fe, and As transformation. The abundances of Klebsiella, Rhizobium, Thiothrix, Sphingomonas, Citrobacter, Desulfomicrobium, and Rhodopseudomonas were positively related to DOC, which implied that DOC might be an important electron donor for diazotrophs in groundwater.
Fig. 3. Relationship between geochemical parameters and diazotrophic communities.
A DNA level. B RNA level. The symbol * indicates Pearson correlation with significance (*, **, and *** represent p < 0.05, 0.01, and 0.001, respectively).
Distribution, composition, and potential interconnected biogeochemical networks of the nitrogenase family in groundwater
Metagenomics and metatranscriptomics were obtained from groundwater samples G1, G2, G27, and G30 and 2.4–4.2 × 107 and 2.3–3.4 × 107 trimmed reads were obtained for the metagenomic and metatranscriptomic datasets, respectively (Table S7). For the potential N2 fixation function of the microbial communities, the relative abundances of KEGG-annotated genes encoding Nif, Vnf, and Anf are shown in Fig. S7. Based on the metagenomic analysis, 17 (sub)nitrogenases were annotated in groundwater, Nif (nifA, nifU, nifV, nifB, nifK, nifD, nifH, nifZ, nifN, nifQ, nifW, nifT, nifHD1, and nifHD2) and Anf (anfG). N2 fixation genes were more abundant in the oxidizing area than in the reducing area (Fig. S7A). Metatranscriptomic annotation results found 19 (sub)nitrogenases, including traditional Nif (nifA, nifU, nifL, nifD, nifE, nifK, nifB, nifN, nifV, nifH, nifX, nifQ, nifZ, nifW, nifT, nifHD1, and nifHD2), selective nitrogenase Anf (anfG), and Vnf (vnfH) (Fig. S7B). Contrasting the metagenomic results, the transcriptional nitrogenases in groundwater were mainly distributed in the reducing area, except for vnfH.
Pearson correlations between nitrogenase abundances and other critical functional genes of N, S, CH4, Fe, and As metabolism processes suggested that N2 fixation might be more positively linked to other biogeochemical cycles at the transcriptional level (Figs. 4 and S8). For example, most of the N2 fixation genes were positively associated with those related to denitrification (nirS and norB) and dissimilatory SO42− reduction (aprB, dsrA, and dsrB) at the DNA level (Fig. S8A and Table S9). By contrast, the transcriptional abundances of nitrogenase clusters were strongly correlated with nitrification (amoA, amoB, amoC, nxrA, and nxrB), denitrification (nirS), dissimilatory SO42− reduction and oxidation (sat, aprA, aprB, dsrA, and dsrB), sulfide oxidation (fccb), CH4 oxidation (pmoA, pmoB, and pmoC), Fe(II) transport (feoA and feoB), As(III) oxidation (aoxB), and As(III) pumping (arsB) (Fig. 4A and Table S10). Moreover, the co-occurrence/expression network analysis of metabolic modules also implied that active diazotrophs were associated more with other biogeochemical processes than native diazotrophs, consistent with the functional gene abundances described above (Figs. 4B and S8B).
Fig. 4. Metabolic potential and genomic bins related to N2 fixation and N/S/Fe/CH4/As cycles in groundwater.
A Pearson correlation analysis between nitrogenase abundances and geochemical parameters/other essential functional genes of N, S, Fe, CH4, and As cycling identified by the KEGG Orthologs database based on metatranscriptome. B The co-expression network of N2 fixation and other biogeochemical processes were analyzed by the KEGG module database (Pearson correlations r > |0.6| and p < 0.05). C Taxonomic distribution and metabolic potential of the bacterial medium-quality metagenomic bins (completeness > 50% and contamination < 10%). For the taxonomy of the bins, “c” stands for class level, and “g” stands for genus level.
The metagenomic datasets produced 37 bacterial and two archaeal medium-quality metagenome-assembled genomes (MAGs) with 51.8 – 98.1% completeness, 0 – 8.5% contamination, and 33.4 – 72.5% GC content (Table. S11). Of these genomes, 19 bacterial MAGs spanning nine different phyla (Acidobacteriota, Bacteroidota, Desulfobacterota, Gemmatimonadota, KSB1, Methylomirabilota, Nitrospirota, Omnitrophota, and Pseudomonadota) carried critical genes involved in N2 fixation (Fig. 4C). A keystone MAG (Thermodesulfovibrionia, bin.16) with a complete nitrogenase also possessed genes associated with denitrification (norBC), organic N to NH4+ (gdhA), polysulfide to sulfide (hydAB), dissimilatory SO42− oxidation and reduction (sat, cysCH, and dsrAB), Fe(II) transport (feoA and feoB), and As(V) detoxification (arsBC). In addition, two keystone MAGs (Thermodesulfovibrionia_bin 16 and Rhodothermia_bin 47) implied potential coupling between N2 fixation and methanogenesis based on the KEGG module annotation (Fig. 4C).
Rates of near in situ N2 fixation in field groundwater and N2-fixer enrichment and isolates
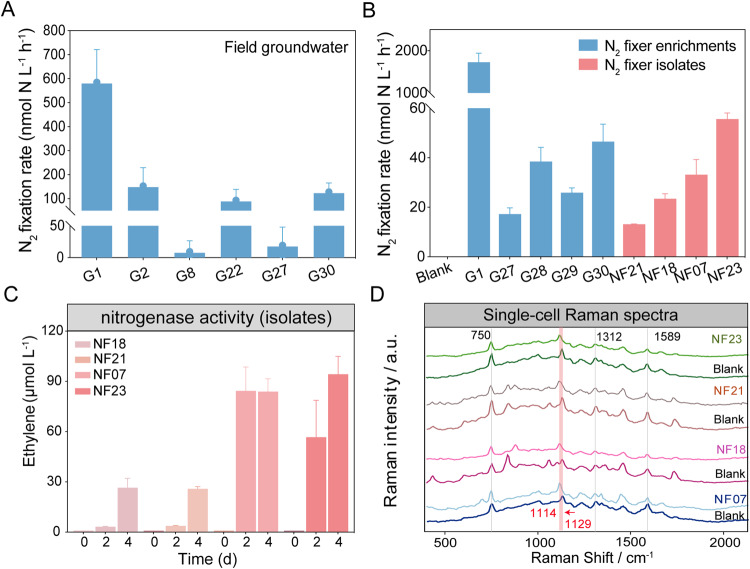
N2 fixation was active in both the oxidizing and reducing areas. For most incubation experiments of field groundwater, both Δδ15N (‰) and 15N concentrations of PN + TDN showed good linearity in the incubation time series R2 > 0.964 (Fig. S9 and Table S12). N2 fixation rates varied from 9.5 to 585.4 nmol N L−1 h−1, with a mean value of 165.0 nmol N L−1 h−1 (Fig. 5A). N2 fixation activity was positively correlated with ORP (p < 0.01), NO3– (p < 0.001), TN (p < 0.01), and nifH/16S rRNA (p < 0.001) (Fig. S4B). After 40 days of incubation, nitrogenase activities of groundwater ranged from 7.0 to 69.7 nmol ethylene L−1 d−1 (Fig. S10). N2-fixer enrichment of groundwater showed high activity, from 17.3 to 1728.4 nmol N g−1 h−1 (Fig. 5B). The highest N2 fixation activity was found in the G1 N2-fixer enrichment, in congruence with the field result. Six N2-fixing strains, designated NF07, NF18, NF21, NF23, E1, and A3, were isolated from groundwater samples. BLAST analysis of the 16S rRNA gene sequences of these strains indicated that four belonged to Klebsiella, and the remaining two belonged to Ensifer and Rhodococcus (Fig. S11). All three of these genera were found in the 16S rRNA gene amplicon sequencing analysis of in situ groundwater and N2-fixer enrichment (Fig. S12). The acetylene reduction assay showed that the nitrogenase activity rates of the strains ranged from 7.8 to 785.2 μmol L−1 day−1 (Figs. 5C and S13). The four active strains were selected to determine their ability to fix 15N2 intracellularly using the 15N isotope tracer technique. The N2 fixation rates ranged from 13.2 to 55.6 nmol N g−1 h−1 (Fig. 5B). In addition, 15N2-induced shifts in the RR band of cytochrome c are a sensitive indicator of N2 fixation [58]. Raman spectra of the four strains under 15N2 incubation showed that the 1129 cm−1 band (C–N stretch) at the blank shifted clearly to 1114 cm−1 at NF07, NF18, NF21, and NF23, confirming the substitution of 14N with 15N in the C–N bond (Fig. 5D). These results demonstrated diazotroph activity in groundwater.
Fig. 5. N2 fixation rates in field groundwater and N2-fixer enrichments and isolates.
The verified function of N2 fixation in the (A) field groundwater and (B) N2-fixer enrichments and isolates (detected by the 15N2 tracing incubation). The activities of N2-fixer isolates were also analyzed using the (C) acetylene reduction method and (D) single-cell resonance Raman spectroscopy. 15N2-induced shifts in the resonance Raman band of Cyt c are a sensitive and robust indicator of N2 fixation. Single-cell Raman spectra from four isolates (D) indicate that the 1129 cm−1 band (C-N stretch) shifted markedly to 1114 cm−1 under 15N2 incubation conditions, implying that it is the substitution of light 14N with heavier 15N in the C-N bond resulting in a decrease in the vibrational frequency of the C-N stretch. The pink shading indicates the 15N2-induced shifts in the resonance Raman spectra.
Discussion
Diazotrophs inhabit groundwater systems
Direct evidence from our integrated 15N isotope tracing, acetylene-reduction assays, and RNA-based nifH gene analysis results indicated that many active diazotrophs inhabit groundwater, although other processes, including abiotic processes or biological denitrification and anaerobic ammonia oxidation, could potentially lead to the overestimation of N2 fixation rates. However, natural groundwater isotopic fractionation reported in previous studies has not approached the isotopic increments measured in this study (Fig. S9 and Table S12) [62–64]. Our data support N2 fixation in groundwater. In addition, ethylene production rates from acetylene-reduction assays were lower than the N2 fixation rates determined through the 15N2 tracing method. This may be attributed to the direct and/or indirect inhibitory effect of acetylene on N2 fixation activity [65–70]. We also found that the average N2 fixation rate was much higher than those reported for oceans and eutrophic estuaries such as the southern Indian Ocean [71], Arctic Ocean [72], North Pacific [73], North Atlantic Ocean [74], Taiwan Strait [75], South China Sea [76], eastern Arabian Sea [77], Kishon River [78], and Cochin Estuary [79], and was comparable to those reported for eutrophic sediments [4, 80, 81]. We hypothesize that excess N2 [1, 36], diverse electron donors [82], and coupling with other biogeochemical processes [42] in groundwater are conducive to diazotrophic activity. The huge difference in N2 fixation rates may be due to the higher PN concentrations (0.2–1 µM) in groundwater [30], compared to the open ocean (average, 0.2–0.3 µM) [83, 84]. Correspondingly, the diversity (Shannon index) of diazotrophs in the groundwater was higher than that in soils and marine surface water [85, 86]. Previous studies proposed that micro-hypoxic environments within particles and biofilms could be a suitable niche for diazotrophs under various redox conditions [1, 87, 88]. Thus, the patchy distribution of N2 fixation rates in oxidizing and reducing conditions may have been caused by the heterogeneity of suspended particles, similar to marine environments [50, 89]. The lower abundance of diazotrophs in groundwater, with N2 fixation rates similar to those of eutrophic estuarine sediments [10, 80, 81, 90], suggests that diazotrophs may have a more diverse and abundant energy source in groundwater.
A comprehensive set of nifH sequences obtained from amplicons, metagenomes, and metatranscriptomes revealed that groundwater-associated diazotrophs were predominately heterotrophic bacteria (Cluster I, aerobic or facultatively anaerobic) (Figs. 2C and S5) [91]. The richer DOC in groundwater may provide a favorable environment for heterotrophic diazotrophs [30, 44, 92]. Recent studies have found that heterotrophic bacterial diazotrophs were more abundant than cyanobacterial diazotrophs in marine environments, highlighting the importance of heterotrophic populations in N2 fixation [93, 94], especially for Gammaproteobacteria and Alphaproteobacteria populations [95]. We found that populations in nifH amplicons, contigs, and N2 fixation enrichments were mainly Gammaproteobacteria and Alphaproteobacteria (Figs. S5, S12). For example, the heterotrophic diazotrophs Klebsiella oxytoca and Ensifer adhaerens cultured from groundwater are Gammaproteobacteria and Alphaproteobacteria. Klebsiella is also an active heterotrophic diazotroph in estuarine environments [96]. Studies have suggested that heterotrophic diazotrophs are widely distributed in seawater through multiple metabolic pathways, such as denitrification and ammonification [95]. The results of our near in situ investigation and laboratory-scale cultures confirmed that active diazotrophs inhibit groundwater systems.
In conjunction with our recent findings, the highest near in situ rates of N2 fixation and NH4+ consumption were observed simultaneously in the oxidizing area (G1) [30]. Due to the lower rates of mineralization and DNRA [30], we propose that N2 fixation is an essential source of N in NH4+-depleted groundwater, promoting N transformation [28, 97]. N2 fixation activity in groundwater samples with 211.2 μM NH4+ implies that diazotrophs fix N2 for reasons other than to meet their own N demand [98], similar to previous findings in aquatic environments [3, 19, 99] and for heterotrophic isolates [95]. Furthermore, there was evidence that N2 fixation could balance the diazotrophic intracellular redox state by removing excess electrons and energy, serving a similar purpose to CO2 fixation [3, 50, 98, 100]. This notion is supported by the positive correlation between diazotrophs and electron donors [Fe(II) and As (III)] found in this study. Thus, N2 fixation in oxidizing groundwater with low NH4+ concentrations sustains available N for microorganisms. In reducing areas with high NH4+ levels, diazotrophs may regulate ideal redox conditions through N2 fixation without synthesizing enzymes with expected functions (redox balancing) [95, 98]. In addition to organic N mineralization, DNRA, and surface input [30], future work on the sources of N in groundwater systems should consider the influence of N2 fixation. Continued spatiotemporal variability studies are needed to comprehensively understand the ecological functions of N2 fixation in aquifers.
Environmental implications of N2 fixation in groundwater systems
Due to the varied hydrogeochemical conditions, diverse microbes could catalyze C, N, S, As, and Fe-related biogeochemical processes in groundwater [32]. Thus, N2 fixation might be coupled with various catabolic processes, contributing to N flow in the groundwater microbiome [101]. Our study showed that N2 fixation was closely linked to nitrification, denitrification, SO42− reduction, sulfide oxidation, CH4 oxidation, As(III) oxidation and pumping, and Fe(II) transport in a typical groundwater system.
N2 fixation and nitrification are the opposite of NH4+ transformation. In this study, N2 fixation was positively associated with nitrification and comammox based on metatranscriptomic analysis (Fig. 4A, B), congruent with the observation that nitrification potentially counteracted the inhibition of NH4+ on diazotrophs, sustaining N2 fixation activity [99, 102]. This finding supported our recent finding that the highest nitrification rates were present in NH4+-deficient groundwater samples (G1 and G2) [30] with more active N2 fixation (Fig. 5A). In addition, oxygen consumption by nitrifiers supports anaerobic microbial metabolism [1], which may be a survival pathway for diazotrophs. Aerobic methanotrophs may also contribute to oxygen depletion. Some aerobic methanotrophs also fix N2. For example, genus Methylocella in the nifH amplicons in our study was found with active CH4 consumers widespread in terrestrial environments [103, 104]. We also recovered a medium-quality MAG affiliated with Gemmatimonadota that was predicted to fix N2 and oxidize CH4 in the G27 groundwater sample (reducing aquifer); Gemmatimonadota has been identified as a fourth phylum potentially capable of aerobic methanotrophy [105]. Besides methanotrophs, diazotrophs found in the current study, such as Rhizobium and Bradyrhizobium, can utilize the methanol produced by methanotrophs [106]. Together, these findings may explain the correlation between N2 fixation and CH4 oxidation in groundwater (Fig. 4A, B) [106].
The positive influence of organic matter (OM) on N2 fixation was probably due to the supplementation of energy for heterotrophic diazotrophs [107]. We also found a significant influence of DOC on diazotrophic communities. In aphotic conditions, the addition of DOM such as free amino acids, glucose, and ATP stimulated N2 fixation in enrichment experiments [50, 89]. In addition to OM metabolism, other biological processes such as SO42− reduction [2] and S oxidation [23], may also drive N2 fixation in groundwater. Putative sulfate-reducing bacteria, including Desulfomicrobium, Desulfuromonas, and Pseudomonas [14, 108], were identified as active diazotrophs in the present study. ATP production by SO42− reduction could partially support N2 fixation [20, 109]. In addition, diazotrophs have been found to use S as a terminal electron acceptor/donor [18, 23]. These factors account for the positive correlation between N2 fixation and SO42− reduction and sulfide oxidation in our results and similar previous findings in benthic and estuarine environments [21]. A recent study found that As-dependent N2 fixation was also widespread in high-As tailings and soils [101]. In our study, diazotrophs were positively correlated with As(III)-oxidizing and As(III)-pumping microbes (aoxB and arsB genes) in groundwater (Fig. 4) characterized by high As concentrations [47, 110]. In addition, members of Hydrogenophaga, an active diazotroph found in this study (Fig. 2D), may participate in As(III) oxidation [111], further refining the coupling between As and N cycling in high-As groundwater [32].
In this study, N2 fixation was closely related to dissimilatory Fe(III) reduction. We found a positive correlation between N2 fixers (abundance and community composition) and Fe(II) concentrations or Fe(II) transport genes (Figs. 1B, 3, 4A, and S6). Some diazotrophs identified in this study have been found with Fe(III) reduction, including Azotobacter, Bacillus, Desulfuromonas, Pseudomonas, and Klebsiella [112–114]. One MAG (Thermodesulfovibrionia, bin 16) found by metagenomic binning may be capable of Fe, S, and As-dependent N2 fixation according to the functional gene or module analysis (Fig. 4C), suggesting that diazotrophs in groundwater also use Fe(III)/ SO42−/As(III) as electron acceptors [115]. Thermodesulfovibrionia was also found in nifH gene contigs in the metagenome and metatranscriptome (Fig. S5). Thermodesulfovibrionia has been reported to dominate reducing NH4+-rich groundwater and may be involved in N turnover and Fe(III) reduction [42, 116]. During organic matter decomposition, Fe(III) is usually reduced before sulfate, yielding 228 kJ/mol energy per reaction [117, 118]. Relationships between N2 fixation rates and Fe(III) reduction and Fe(II) concentrations have also been found in pure cultures (from our unpublished data), as well as in coastal sediments, soils, and wetlands [10, 22, 81], perhaps because Fe(III)-reducing bacteria can fix N2 directly and provide energy for N2 fixation using H2 or organic C as electron donors [20, 117].
Overall, our results indicate that diazotrophs were active in groundwater and may interact positively with other microorganisms. N2 fixation may be coupled with other elemental cycles in groundwater both among microbial species and as an intra-species process. Further studies of the correlated mechanisms of N2 fixation and C/N/S/As/Fe metabolism in groundwater are warranted. The contribution of N2 fixation to groundwater systems globally should also be explored. It should be possible to culture novel diazotrophs and nitrogenases from groundwater.
Conclusion
Our study demonstrates the activity, abundance, and distribution of diazotrophs in groundwater, indicating that N2 fixation was sufficiently active and abundant to affect biogeochemical cycling in groundwater systems. The majority of nifH communities in amplicons, metagenomes, and metatranscriptomes were aerobic or facultative anaerobic diazotrophs, supporting the expression of nifH genes throughout varied redox groundwater conditions. Moreover, a tight correlation was found between N2 fixation and various biogeochemical cycles, such as nitrification, SO42− reduction, sulfide oxidation, Fe(II) transport, CH4 oxidation, As(III) oxidation, and As-resistant. Here, we identified groundwater systems as neglected habitats for diazotrophs and provided insight into N2 fixation with distinct geochemical factors and N/CH4/S/As/Fe cyclings. Further understanding of the synergistic mechanisms of N2 fixation and other biogeochemical processes is necessary.
Supplementary information
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (Grant No 42177068, 91851115, 91851209, and 91851101).
Author contributions
Conceptualization: PL, XL, and HW. Sample collection and methodology: HW, XL, L-LH, PL, YW, and ZJ. Analysis and writing-original draft preparation: XL and HW. Writing-review and editing and funding acquisition: PL, LC, and S-JK. 15N analysis: L-LH and S-JK. Single-cell resonance Raman-15N2 SIP analysis: KY and LC.
Data availability
The raw data have been submitted to the NCBI database under BioProject numbers PRJNA937386, PRJNA996345, PRJNA996367, PRJNA882225, and PRJNA884812.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41396-023-01513-x.
References
- 1.Mosley O, Gios E, Close M, Weaver L, Daughney C, Handley K. Nitrogen cycling and microbial cooperation in the terrestrial subsurface. ISME J. 2022;16:2561–73.. doi: 10.1038/s41396-022-01300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong X, Zhang C, Peng Y, Zhang H-X, Shi L-D, Wei G, et al. Phylogenetically and catabolically diverse diazotrophs reside in deep-sea cold seep sediments. Nat Commun. 2022;13:4885. doi: 10.1038/s41467-022-32503-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gier J, Sommer S, Löscher C, Dale A, Schmitz R, Treude T. Nitrogen fixation in sediments along a depth transect through the Peruvian oxygen minimum zone. Biogeosciences. 2016;13:4065–80.. [Google Scholar]
- 4.Tian L, Yan Z, Wang C, Xu S, Jiang H. Habitat heterogeneity induces regional differences in sediment nitrogen fixation in eutrophic freshwater lake. Sci Total Environ. 2021;772:145594. doi: 10.1016/j.scitotenv.2021.145594. [DOI] [PubMed] [Google Scholar]
- 5.Capone DG, Burns JA, Montoya JP, Subramaniam A, Mahaffey C, Gunderson T, et al. Nitrogen fixation by Trichodesmium spp.: An important source of new nitrogen to the tropical and subtropical North Atlantic Ocean. Global Biogeochem Cy. 2005;19:GB2024.
- 6.Foster RA, Kuypers MMM, Vagner T, Paerl RW, Musat N, Zehr JP. Nitrogen fixation and transfer in open ocean diatom–cyanobacterial symbioses. ISME J. 2011;5:1484–93. doi: 10.1038/ismej.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zehr J, Shilova I, Farnelid H, Muñoz Marin M, Turk-Kubo K. Unusual marine unicellular symbiosis with the nitrogen-fixing cyanobacterium UCYN-A. Nat Microbiol. 2016;2:16214. doi: 10.1038/nmicrobiol.2016.214. [DOI] [PubMed] [Google Scholar]
- 8.Steenhoudt O, Vanderleyden J. Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: Genetic, biochemical and ecological aspects. FEMS Microbiol Rev. 2000;24:487–506. doi: 10.1111/j.1574-6976.2000.tb00552.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Ward BB, Sigman DM. Global nitrogen cycle: Critical enzymes, organisms, and processes for nitrogen budgets and dynamics. Chem Rev. 2020;120:5308–51.. doi: 10.1021/acs.chemrev.9b00613. [DOI] [PubMed] [Google Scholar]
- 10.Hou L, Wang R, Yin G, Liu M, Zheng Y. Nitrogen fixation in the intertidal sediments of the Yangtze Estuary: Occurrence and environmental implications. J Geophys Res-Biogeo. 2018;123:936–44.. [Google Scholar]
- 11.Saiz E, Sgouridis F, Drijfhout FP, Ullah S. Biological nitrogen fixation in peatlands: Comparison between acetylene reduction assay and 15N2 assimilation methods. Soil Biol Biochem. 2019;131:157–65.. [Google Scholar]
- 12.Tsoy OV, Ravcheev DA, Čuklina J, Gelfand MS. Nitrogen fixation and molecular oxygen: Comparative genomic reconstruction of transcription regulation in Alphaproteobacteria. Front Microbiol. 2016;7:1343. doi: 10.3389/fmicb.2016.01343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyd E, Peters J. New insights into the evolutionary history of biological nitrogen fixation. Front Microbiol. 2013;4:201. doi: 10.3389/fmicb.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newell S, Pritchard K, Foster S, Fulweiler W. Molecular evidence for sediment nitrogen fixation in a temperate New England estuary. PeerJ. 2016;4:e1615. doi: 10.7717/peerj.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reed S, Cleveland C, Townsend A. Functional ecology of free-living nitrogen fixation: A contemporary perspective. Annu Rev Ecol Evol Syst. 2011;42:489–512. [Google Scholar]
- 16.Houlton BZ, Wang Y-P, Vitousek PM, Field CB. A unifying framework for dinitrogen fixation in the terrestrial biosphere. Nature. 2008;454:327–30. doi: 10.1038/nature07028. [DOI] [PubMed] [Google Scholar]
- 17.Smercina D, Evans S, Friesen M, Tiemann L. To fix or not to fix: Controls on free-living nitrogen-fixation in the rhizosphere. Appl Environ Microbiol. 2019;85:e02546–18.. doi: 10.1128/AEM.02546-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapili B, Barnett S, Buckley D, Dekas A. Evidence for phylogenetically and catabolically diverse active diazotrophs in deep-sea sediment. ISME J. 2020;14:971–83.. doi: 10.1038/s41396-019-0584-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertics V, Löscher C, Salonen I, Dale A, Gier J, Schmitz RA, et al. Occurrence of benthic microbial nitrogen fixation coupled to sulfate reduction in the seasonally hypoxic Eckernförde Bay, Baltic Sea. Biogeosciences. 2013;10:1243–58.. [Google Scholar]
- 20.Gier J, Löscher C, Dale A, Sommer S, Lomnitz U, Treude T. Benthic dinitrogen fixation traversing the oxygen minimum zone off Mauritania (NW Africa) Front Mar Sci. 2017;4:390. [Google Scholar]
- 21.Fulweiler W, Brown S, Nixon S, Jenkins B. Evidence and a conceptual model for the co-occurrence of nitrogen fixation and denitrification in heterotrophic marine sediments. Mar Ecol Prog Ser. 2013;482:57–68. [Google Scholar]
- 22.Jia R, Wang K, Li L, Qu Z, Shen W, Qu D. Abundance and community succession of nitrogen-fixing bacteria in ferrihydrite enriched cultures of paddy soils is closely related to Fe(III)-reduction. Sci Total Environ. 2020;720:137633. doi: 10.1016/j.scitotenv.2020.137633. [DOI] [PubMed] [Google Scholar]
- 23.Sun X, Kong T, Häggblom M, Kolton M, Li F, Dong Y, et al. Chemolithoautotropic diazotrophy dominates the nitrogen fixation process in mine tailings. Environ Sci Technol. 2020;54:6082–93.. doi: 10.1021/acs.est.9b07835. [DOI] [PubMed] [Google Scholar]
- 24.Alley W, Healy R, LaBaugh J, Reilly T. Flow and storage in groundwater systems. Science. 2002;296:1985–90. doi: 10.1126/science.1067123. [DOI] [PubMed] [Google Scholar]
- 25.Griebler C, Lueders T. Microbial biodiversity in groundwater ecosystems. Freshw Biol. 2009;54:649–77.. [Google Scholar]
- 26.Nai H, Xin J, Liu Y, Zheng X, Lin Z. Distribution and molecular chemodiversity of dissolved organic nitrogen in the vadose zone-groundwater system of a fluvial plain, northern China: Implications for understanding its loss pathway to groundwater. Sci Total Environ. 2020;723:137928. doi: 10.1016/j.scitotenv.2020.137928. [DOI] [PubMed] [Google Scholar]
- 27.Jiang Z, Shen X, Shi B, Cui M, Wang Y, Li P. Arsenic mobilization and transformation by ammonium-generating bacteria isolated from high arsenic groundwater in Hetao Plain, China. Int J Environ Res Public Health. 2022;19:9606. doi: 10.3390/ijerph19159606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar S, Herrmann M, Blohm A, Hilke I, Frosch T, Trumbore S, et al. Thiosulfate- and hydrogen-driven autotrophic denitrification by a microbial consortium enriched from groundwater of an oligotrophic limestone aquifer. FEMS Microbiol Ecol. 2018; 94; 10.1093/femsec/fiy141. [DOI] [PubMed]
- 29.Wang S, Radny D, Huang S, Zhuang L, Zhao S, Berg M, et al. Nitrogen loss by anaerobic ammonium oxidation in unconfined aquifer soils. Sci Rep. 2017;7:40173. doi: 10.1038/srep40173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han L-L, Wang H, Ge L, Xu M, Tang J-M, Li L, et al. Transition of source/sink processes and fate of ammonium in groundwater along with redox gradients. Water Res. 2023;231:119600. doi: 10.1016/j.watres.2023.119600. [DOI] [PubMed] [Google Scholar]
- 31.Xiong Y, Du Y, Deng Y, Ma T, Wang Y. Feammox in alluvial-lacustrine aquifer system: Nitrogen/iron isotopic and biogeochemical evidences. Water Res. 2022;222:118867. doi: 10.1016/j.watres.2022.118867. [DOI] [PubMed] [Google Scholar]
- 32.Li P, Jiang Z, Wang Y, Deng Y, Van Nostrand J, Yuan T, et al. Analysis of the functional gene structure and metabolic potential of microbial community in high arsenic groundwater. Water Res. 2017;123:268–76.. doi: 10.1016/j.watres.2017.06.053. [DOI] [PubMed] [Google Scholar]
- 33.Xin J, Liu Y, Chen F, Duan Y, Wei G, Zheng X, et al. The missing nitrogen pieces: A critical review on the distribution, transformation, and budget of nitrogen in the vadose zone-groundwater system. Water Res. 2019;165:114977. doi: 10.1016/j.watres.2019.114977. [DOI] [PubMed] [Google Scholar]
- 34.Zhu G, Wang S, Li Y, Zhuang L, Zhao S, Wang C, et al. Microbial pathways for nitrogen loss in an upland soil. Environ Microbiol. 2018;20:1723–38.. doi: 10.1111/1462-2920.14098. [DOI] [PubMed] [Google Scholar]
- 35.Zhou W, Xia L, Yan X. Vertical distribution of denitrification end-products in paddy soils. Sci Total Environ. 2017;576:462–71.. doi: 10.1016/j.scitotenv.2016.10.135. [DOI] [PubMed] [Google Scholar]
- 36.Mosley OE, Gios E, Weaver L, Close M, Daughney C, van der Raaij R, et al. Metabolic diversity and aero-tolerance in anammox bacteria from geochemically distinct aquifers. mSystems. 2022;7:e01255–21.. doi: 10.1128/msystems.01255-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang Z, Li P, Wang Y, Liu H, Wei D, Yuan C, et al. Arsenic mobilization in a high arsenic groundwater revealed by metagenomic and Geochip analyses. Sci Rep. 2019;9:12972. doi: 10.1038/s41598-019-49365-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong Y, Sanford RA, Connor L, Chee-Sanford J, Wimmer BT, Iranmanesh A, et al. Differential structure and functional gene response to geochemistry associated with the suspended and attached shallow aquifer microbiomes from the Illinois Basin, IL. Water Res. 2021;202:117431. doi: 10.1016/j.watres.2021.117431. [DOI] [PubMed] [Google Scholar]
- 39.Méheust R, Castelle C, Carnevali P, Farag I, He C, Chen L, et al. Groundwater Elusimicrobia are metabolically diverse compared to gut microbiome Elusimicrobia and some have a novel nitrogenase paralog. ISME J. 2020;14:2907–22.. doi: 10.1038/s41396-020-0716-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bae H-S, Rash B, Rainey F, Nobre M, Tiago I, da Costa M, et al. Description of Azospira restricta sp. nov., a nitrogen-fixing bacterium isolated from groundwater. Int J Syst Evol Microbiol. 2007;57:1521–26.. doi: 10.1099/ijs.0.64965-0. [DOI] [PubMed] [Google Scholar]
- 41.Pedron R, Luchi E, Albiac MA, Di Cagno R, Catorci D, Esposito A, et al. Mesorhizobium comanense sp. nov., isolated from groundwater. Int J Syst Evol Microbiol. 2021; 71; 10.1099/ijsem.0.005131. [DOI] [PubMed]
- 42.Xiu W, Wu M, Nixon SL, Lloyd JR, Bassil NM, Gai R, et al. Genome-resolved metagenomic analysis of groundwater: Insights into arsenic mobilization in biogeochemical interaction networks. Environ Sci Technol. 2022;56:10105–19.. doi: 10.1021/acs.est.2c02623. [DOI] [PubMed] [Google Scholar]
- 43.Chen M, Lu Y, Jiao N, Tian J, Kao S-J, Zhang Y. Biogeographic drivers of diazotrophs in the western Pacific Ocean. Limnol Oceanogr. 2019;64:1403–21.. [Google Scholar]
- 44.Wang Y, Zhang G, Wang H, Cheng Y, Liu H, Jiang Z, et al. Effects of different dissolved organic matter on microbial communities and arsenic mobilization in aquifers. J Hazard Mater. 2021;411:125146. doi: 10.1016/j.jhazmat.2021.125146. [DOI] [PubMed] [Google Scholar]
- 45.Poly F, Monrozier LJ, Bally R. Improvement in the RFLP procedure for studying the diversity of nifH genes in communities of nitrogen fixers in soil. Res Microbiol. 2001;152:95–103. doi: 10.1016/s0923-2508(00)01172-4. [DOI] [PubMed] [Google Scholar]
- 46.Uritskiy GV, DiRuggiero J, Taylor J. MetaWRAP—a flexible pipeline for genome-resolved metagenomic data analysis. Microbiome. 2018;6:158. doi: 10.1186/s40168-018-0541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang Z, Zhong S, Shen X, Cui M, Wang Y, Li J. Microbially mediated arsenic mobilization in the clay layer and underlying aquifer in the Hetao Basin, Inner Mongolia, China. Sci Total Environ. 2022;836:155597. doi: 10.1016/j.scitotenv.2022.155597. [DOI] [PubMed] [Google Scholar]
- 48.Wang H, Li P, Liu X, Zhang J, Stein LY, Gu J-D. An overlooked influence of reactive oxygen species on ammonia-oxidizing microbial communities in redox-fluctuating aquifers. Water Res. 2023;233:119734. doi: 10.1016/j.watres.2023.119734. [DOI] [PubMed] [Google Scholar]
- 49.Montoya JP, Voss M, Kahler P, Capone DG. A simple, high-precision, high-sensitivity tracer assay for N(inf2) fixation. Appl Environ Microbiol. 1996;62:986–93. doi: 10.1128/aem.62.3.986-993.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu S, Moge D, Wan X, Selden C, Benavides M, Bonnet S, et al. Insights into nitrogen fixation below the euphotic zone: Trials in an oligotrophic marginal sea and global compilation. Biogeosciences Discuss. 2021;2021:1–31.. [Google Scholar]
- 51.Berthelot H, Benavides M, Moisander P, Grosso O, Bonnet S. High Nitrogen fixation rates in the particulate and dissolved pools in the Western Tropical Pacific (Solomon and Bismarck Seas) Geophys Res Lett. 2017;44:8414–23.. [Google Scholar]
- 52.Knapp AN, Sigman DM, Lipschultz F. N isotopic composition of dissolved organic nitrogen and nitrate at the Bermuda Atlantic Time-series Study site. Global Biogeochem Cy. 2005;19:1–15.
- 53.Xu MN, Wu Y, Zheng LW, Zheng Z, Zhao H, Laws EA, et al. Quantification of multiple simultaneously occurring nitrogen flows in the euphotic ocean. Biogeosciences. 2017;14:1021–38.. [Google Scholar]
- 54.Lu Y, Wen Z, Shi D, Lin W, Bonnet S, Dai M, et al. Biogeography of N2 fixation influenced by the western boundary current intrusion in the South China Sea. J Geophys Res: Oceans. 2019;124:6983–96.. [Google Scholar]
- 55.Lu Y, Wen Z, Shi D, Chen M, Zhang Y, Bonnet S, et al. Effect of light on N2 fixation and net nitrogen release of Trichodesmium in a field study. Biogeosciences. 2018;15:1–12. [Google Scholar]
- 56.Dabundo R, Lehmann MF, Treibergs L, Tobias CR, Altabet MA, Moisander PH, et al. The contamination of commercial 15N2 gas stocks with 15N–labeled nitrate and ammonium and consequences for nitrogen fixation measurements. PLoS ONE. 2014;9:e110335. doi: 10.1371/journal.pone.0110335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fulweiler RWW, Heiss EM, Rogener MK, Newell SE, Wilhelm SW. Examining the impact of acetylene on N-fixation and the active sediment microbial community. Front Microbiol. 2015;6:418. doi: 10.3389/fmicb.2015.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cui L, Yang K, Li H-Z, Zhang H, Su J-Q, Paraskevaidi M, et al. Functional single-cell approach to probing nitrogen-fixing bacteria in soil communities by resonance raman spectroscopy with 15N2 labeling. Anal Chem. 2018;90:5082–89.. doi: 10.1021/acs.analchem.7b05080. [DOI] [PubMed] [Google Scholar]
- 59.Cui L, Yang K, Zhou G, Huang W. Surface-enhanced raman spectroscopy combined with stable isotope probing to monitor nitrogen assimilation at both bulk and single-cell level. Anal Chem. 2017;89:5793–800.. doi: 10.1021/acs.analchem.6b04913. [DOI] [PubMed] [Google Scholar]
- 60.Csardi G, Nepusz T. The Igraph software package for complex network research. Interjournal Complex Systems. 2006;1695:1–9.
- 61.Newman MEJ. Modularity and community structure in networks. Proc Natl Acad Sci USA. 2006;103:8577–82. doi: 10.1073/pnas.0601602103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao Z, Weng H, Guo H. Unraveling influences of nitrogen cycling on arsenic enrichment in groundwater from the Hetao Basin using geochemical and multi-isotopic approaches. J Hydrol. 2021;595:125981. [Google Scholar]
- 63.Aharoni I, Dahan O, Siebner H. Continuous monitoring of dissolved inorganic nitrogen (DIN) transformations along the waste-vadose zone - groundwater path of an uncontrolled landfill, using multiple N-species isotopic analysis. Water Res. 2022;219:118508. doi: 10.1016/j.watres.2022.118508. [DOI] [PubMed] [Google Scholar]
- 64.Carrey R, Ballesté E, Blanch AR, Lucena F, Pons P, López JM, et al. Combining multi-isotopic and molecular source tracking methods to identify nitrate pollution sources in surface and groundwater. Water Res. 2021;188:116537. doi: 10.1016/j.watres.2020.116537. [DOI] [PubMed] [Google Scholar]
- 65.Larmola T, Leppänen SM, Tuittila E-S, Aarva M, Merilä P, Fritze H, et al. Methanotrophy induces nitrogen fixation during peatland development. Proc Natl Acad Sci USA. 2014;111:734–39. doi: 10.1073/pnas.1314284111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oremland Ronald S, Taylor, Barrie F. Inhibition of methanogenesis in marine sediments by acetylene and ethylene: Validity of the acetylene reduction assay for anaerobic microcosms. Appl Microbiol. 1975;30:707–09. doi: 10.1128/am.30.4.707-709.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ho A, Bodelier PLE. Diazotrophic methanotrophs in peatlands: the missing link? Plant Soil. 2015;389:419–23.. [Google Scholar]
- 68.Farías L, Faúndez J, Fernández C, Cornejo M, Sanhueza S, Carrasco C. Biological N2O fixation in the Eastern South Pacific Ocean and marine cyanobacterial cultures. PLoS ONE. 2013;8:e63956. doi: 10.1371/journal.pone.0063956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Desloover J, Roobroeck D, Heylen K, Puig S, Boeckx P, Verstraete W, et al. Pathway of nitrous oxide consumption in isolated Pseudomonas stutzeri strains under anoxic and oxic conditions. Environ Microbiol. 2014;16:3143–52. doi: 10.1111/1462-2920.12404. [DOI] [PubMed] [Google Scholar]
- 70.Hynes RK, Knowles R. Effect of acetylene on autotrophic and heterotrophic nitrification. Can J Microbiol. 1982;28:334–40.. [Google Scholar]
- 71.Shiozaki T, Ijichi M, Kodama T, Takeda S, Furuya K. Heterotrophic bacteria as major nitrogen fixers in the euphotic zone of the Indian Ocean. Global Biogeochem Cy. 2014;28:1096–110.. [Google Scholar]
- 72.Sipler R, Gong D, Baer S, Sanderson M, Roberts Q, Mulholland M, et al. Preliminary estimates of the contribution of Arctic nitrogen fixation to the global nitrogen budget. Limnol Oceanogr Lett. 2017;2:159–66.. [Google Scholar]
- 73.Shiozaki T, Bombar D, Riemann L, Hashihama F, Takeda S, Yamaguchi T, et al. Basin scale variability of active diazotrophs and nitrogen fixation in the North Pacific, from the tropics to the subarctic Bering Sea. Global Biogeochem Cy. 2017;31:996–1009. [Google Scholar]
- 74.Moore M, Mills M, Achterberg E, Geider R, Laroche J, Lucas M, et al. Large-scale distribution of Atlantic nitrogen fixation controlled by iron availability. Nat Geosci. 2009;2:867–71.. [Google Scholar]
- 75.Wen Z, Lin W, Shen R, Hong H, Kao S-J, Shi D. Nitrogen fixation in two coastal upwelling regions of the Taiwan Strait. Sci Rep. 2017;7:17601. doi: 10.1038/s41598-017-18006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen Y-L, Chen H-Y, Tuo S-H, Ohki K. Seasonal dynamics of new production from Trichodesmium N2 fixation and nitrate uptake in the upstream Kuroshio and South China Sea basin. Limnol Oceanogr. 2008;53:1705–21.. [Google Scholar]
- 77.Kumar P, Singh A, Ramesh R, Nallathambi T. N2 fixation in the eastern Arabian sea: Probable role of heterotrophic diazotrophs. Front Mar Sci. 2017;4:80.
- 78.Geisler E, Rahav E, Bar Zeev E. Contribution of heterotrophic diazotrophs to N2 fixation in a eutrophic river: free-living vs. aggregate-associated. Front Microbiol. 2022;13:779820. doi: 10.3389/fmicb.2022.779820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jabir T, Vipindas PV, Jesmi Y, Valliyodan S, Parambath PM, Singh A, et al. Nutrient stoichiometry (N:P) controls nitrogen fixation and distribution of diazotrophs in a tropical eutrophic estuary. Mar Pollut Bull. 2020;151:110799. doi: 10.1016/j.marpolbul.2019.110799. [DOI] [PubMed] [Google Scholar]
- 80.Wang R, Li X, Hou L, Liu M, Zheng Y, Yin G, et al. Nitrogen fixation in surface sediments of the East China Sea: Occurrence and environmental implications. Mar Pollut Bull. 2018;137:542–48.. doi: 10.1016/j.marpolbul.2018.10.063. [DOI] [PubMed] [Google Scholar]
- 81.Liu C, li N, Shao X, Gao D, Xia J, Cui Q, et al. Effects of coastal marsh conversion to shrimp aquaculture ponds on sediment nitrogen fixation. Front Mar Sci. 2022;9:1034145. [Google Scholar]
- 82.Glodowska M, Stopelli E, Straub D, Vu Thi D, Trang PTK, Viet PH, et al. Arsenic behavior in groundwater in Hanoi (Vietnam) influenced by a complex biogeochemical network of iron, methane, and sulfur cycling. J Hazard Mater. 2021;407:124398. doi: 10.1016/j.jhazmat.2020.124398. [DOI] [PubMed] [Google Scholar]
- 83.Karl DM, Church MJ. Ecosystem structure and dynamics in the north Pacific Subtropical Gyre: New views of an old ocean. Ecosystems. 2017;20:433–57.. [Google Scholar]
- 84.Karl D, Letelier R, Tupas L, Dore J, Christian J, Hebel D. The role of nitrogen fixation in biogeochemical cycling in the subtropical North Pacific Ocean. Nature. 1997;388:533–38.. [Google Scholar]
- 85.Zhu C, Friman V-P, Li L, Xu Q, Guo J, Guo S, et al. Meta-analysis of diazotrophic signatures across terrestrial ecosystems at the continental scale. Environ Microbiol. 2022;24:2013–28.. doi: 10.1111/1462-2920.15984. [DOI] [PubMed] [Google Scholar]
- 86.Messer L, Brown M, Furnas M, Carney R, McKinnon A, Seymour J. Diversity and activity of diazotrophs in Great Barrier reef surface waters. Front Microbiol. 2017;8:967. doi: 10.3389/fmicb.2017.00967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tan E, Hsu T-C, Huang X, Lin H-J, Kao S-J. Nitrogen transformations and removal efficiency enhancement of a constructed wetland in subtropical Taiwan. Sci Total Environ. 2017;601-602:1378–88.. doi: 10.1016/j.scitotenv.2017.05.282. [DOI] [PubMed] [Google Scholar]
- 88.Farnelid H, Turk-Kubo K, Ploug H, Ossolinski J, Collins J, Van Mooy B, et al. Diverse diazotrophs are present on sinking particles in the North Pacific subtropical gyre. ISME J. 2018;13:170–82.. doi: 10.1038/s41396-018-0259-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bonnet S, Dekaezemacker J, Turk-Kubo K, Moutin T, Hamersley M, Grosso O, et al. Aphotic N2 fixation in the eastern tropical South Pacific Ocean. PLoS ONE. 2013;8:e81265. doi: 10.1371/journal.pone.0081265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang F, Lin X, Hu W, Zeng F, He L, Yin KD. Nitrogen cycling processes in sediments of the Pearl River Estuary: Spatial variations, controlling factors, and environmental implications. CATENA. 2021;206:105545. [Google Scholar]
- 91.Moynihan MA, Goodkin NF, Morgan KM, Kho PYY, Lopes dos Santos A, Lauro FM, et al. Coral-associated nitrogen fixation rates and diazotrophic diversity on a nutrient-replete equatorial reef. ISME J. 2022;16:233–46.. doi: 10.1038/s41396-021-01054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zehr J, Capone D. Changing perspectives in marine nitrogen fixation. Science. 2020;368:729. doi: 10.1126/science.aay9514. [DOI] [PubMed] [Google Scholar]
- 93.Delmont TO, Pierella Karlusich JJ, Veseli I, Fuessel J, Eren AM, Foster RA, et al. Heterotrophic bacterial diazotrophs are more abundant than their cyanobacterial counterparts in metagenomes covering most of the sunlit ocean. ISME J. 2022;16:927–36.. doi: 10.1038/s41396-021-01135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Messer LF, Mahaffey C, M Robinson C, Jeffries TC, Baker KG, Bibiloni Isaksson J, et al. High levels of heterogeneity in diazotroph diversity and activity within a putative hotspot for marine nitrogen fixation. ISME J. 2016;10:1499–513. doi: 10.1038/ismej.2015.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bentzon-Tilia M, Severin I, Hansen L, Riemann L. Genomics and ecophysiology of heterotrophic nitrogen-fixing bacteria isolated from estuarine surface water. mBio. 2015;6:e00929–15. doi: 10.1128/mBio.00929-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yousuf J, Thajudeen J, Aneesa PA, Joseph A, Divya PS, Varghese A, et al. Diversity and activity of culturable nitrogen fixing heterotrophic bacteria from estuarine and coastal environments of Southeastern Arabian Sea (SEAS). Reg Stud Mar Sci. 2019; 33:100973.
- 97.Handley K, Verberkmoes N, Steefel C, Williams K, Sharon I, Miller C, et al. Biostimulation induces syntrophic interactions that impact C, S and N cycling in a sediment microbial community. ISME J. 2013;7:800–16. doi: 10.1038/ismej.2012.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bombar D, Paerl R, Riemann L. Marine non-cyanobacterial diazotrophs: moving beyond molecular detection. Trends Microbiol. 2016;24:916–27.. doi: 10.1016/j.tim.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 99.Knapp AN. The sensitivity of marine N2 fixation to dissolved inorganic nitrogen. Front Microbiol. 2012;3:374. doi: 10.3389/fmicb.2012.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McKinlay J, Harwood C. Inaugural Article: carbon dioxide fixation as a central redox cofactor recycling mechanism in bacteria. Proc Natl Acad Sci USA. 2010;107:11669–75. doi: 10.1073/pnas.1006175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li Y, Guo L, Häggblom MM, Yang R, Li M, Sun X, et al. Serratia spp. are responsible for nitrogen fixation fueled by As(III) oxidation, a novel biogeochemical process identified in mine tailings. Environ Sci Technol. 2022;56:2033–43.. doi: 10.1021/acs.est.1c06857. [DOI] [PubMed] [Google Scholar]
- 102.Bertics V, Sohm J, Treude T, Chow C-E, Fuhrman J, Ziebis W. Burrowing deeper into benthic nitrogen cycling: The impact of Bioturbation on nitrogen fixation coupled to sulfate reduction. Mar Ecol Prog Ser. 2010;409:1–15. [Google Scholar]
- 103.Farhan Ul Haque M, Hernández M, Crombie AT, Murrell JC. Identification of active gaseous-alkane degraders at natural gas seeps. ISME J. 2022;16:1705–16.. doi: 10.1038/s41396-022-01211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Farhan Ul Haque M, Crombie AT, Ensminger SA, Baciu C, Murrell JC. Facultative methanotrophs are abundant at terrestrial natural gas seeps. Microbiome. 2018;6:118. doi: 10.1186/s40168-018-0500-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bay SK, Dong X, Bradley JA, Leung PM, Grinter R, Jirapanjawat T, et al. Trace gas oxidizers are widespread and active members of soil microbial communities. Nat Microbiol. 2021;6:246–56.. doi: 10.1038/s41564-020-00811-w. [DOI] [PubMed] [Google Scholar]
- 106.Cui J, Zhang M, Chen L, Zhang S, Luo Y, Cao W, et al. Methanotrophs contribute to nitrogen fixation in emergent macrophytes. Front Microbiol. 2022;13:851424. doi: 10.3389/fmicb.2022.851424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fulweiler RW, Nixon SW, Buckley BA, Granger SL. Reversal of the net dinitrogen gas flux in coastal marine sediments. Nature. 2007;448:180–82. doi: 10.1038/nature05963. [DOI] [PubMed] [Google Scholar]
- 108.Yu X, Zhou J, Song W, Xu M, He Q, Peng Y, et al. SCycDB: A curated functional gene database for metagenomic profiling of sulfur cycling pathways. Mol Ecol Res. 2020;21:924–40.. [Google Scholar]
- 109.Welsh D, Wellsbury P, Bourguès S, De Wit R, Herbert R. Relationship between porewater organic carbon content, sulphate reduction and nitrogen fixation (acetylene reduction) in the rhizosphere of Zostera noltii. Hydrobiologia. 1996;329:175–83.. [Google Scholar]
- 110.Wang Y, Wei D, Li P, Jiang Z, Liu H, Qing C, et al. Diversity and arsenic-metabolizing gene clusters of indigenous arsenate-reducing bacteria in high arsenic groundwater of the Hetao Plain, Inner Mongolia. Ecotoxicology. 2021;30:1680–88.. doi: 10.1007/s10646-020-02305-1. [DOI] [PubMed] [Google Scholar]
- 111.Hoven R, Santini J. Arsenite oxidation by the heterotroph Hydrogenophaga sp. str. NT-14: the arsenite oxidase and its physiological electron acceptor. BBA Bioenergetics. 2004;1656:148–55. doi: 10.1016/j.bbabio.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 112.Li L, Jia R, Qu Z, Li T, Shen W, Qu D. Coupling between nitrogen-fixing and iron(III)-reducing bacteria as revealed by the metabolically active bacterial community in flooded paddy soils amended with glucose. Sci Total Environ. 2020;716:137056. doi: 10.1016/j.scitotenv.2020.137056. [DOI] [PubMed] [Google Scholar]
- 113.Zhang H, Liu F, Zheng S, Chen L, Zhang X, Gong J. The differentiation of iron-reducing bacterial community and iron-reduction activity between riverine and marine sediments in the Yellow River estuary. Mar Life Sci Tech. 2019;2:87–96. [Google Scholar]
- 114.Liu H, Li P, Wang H, Qing C, Tan T, Shi B, et al. Arsenic mobilization affected by extracellular polymeric substances (EPS) of the dissimilatory iron reducing bacteria isolated from high arsenic groundwater. Sci Total Environ. 2020;735:139501. doi: 10.1016/j.scitotenv.2020.139501. [DOI] [PubMed] [Google Scholar]
- 115.Garber AI, Nealson KH, Okamoto A, McAllister SM, Chan CS, Barco RA, et al. FeGenie: A comprehensive tool for the identification of iron genes and iron gene neighborhoods in genome and metagenome assemblies. Front Microbiol. 2020;11:37. [DOI] [PMC free article] [PubMed]
- 116.Yan L, Herrmann M, Kampe B, Lehmann R, Totsche KU, Küsel K. Environmental selection shapes the formation of near-surface groundwater microbiomes. Water Res. 2020;170:115341. doi: 10.1016/j.watres.2019.115341. [DOI] [PubMed] [Google Scholar]
- 117.Lovley DR. Organic matter mineralization with the reduction of ferric iron: A review. Geomicrobiol J. 1987;5:375–99.. [Google Scholar]
- 118.Jones JG, Gardener S, Simon BM. Bacterial reduction of ferric iron in a stratified eutrophic lake. Microbiology. 1983;129:131–39.. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data have been submitted to the NCBI database under BioProject numbers PRJNA937386, PRJNA996345, PRJNA996367, PRJNA882225, and PRJNA884812.