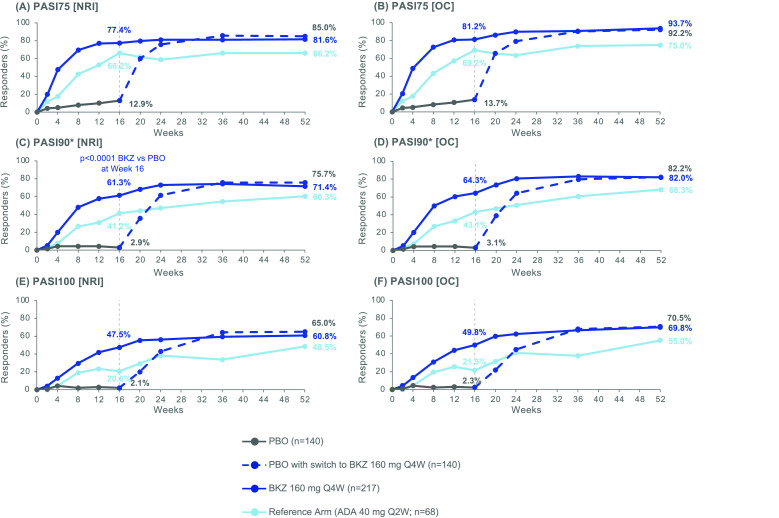
Figure 3.
PASI responders to Week 52. Randomised set, in patients with psoriasis affecting ≥3% BSA at baseline. P value was calculated using a logistic regression model with treatment, bone erosion at baseline and region as stratification factors. The study was not powered for statistical comparisons of ADA with BKZ or PBO. All data points can be found in table format in online supplemental table 6. *PASI90 was a ranked secondary end point. ADA, adalimumab; BKZ, bimekizumab; BSA, body surface area; NRI, non-responder imputation; OC, observed case; PASI75/90/100, Psoriasis Area and Severity Index ≥75%/90%/100% improvement; PBO, placebo; Q2W, every 2 weeks; Q4W, every 4 weeks.