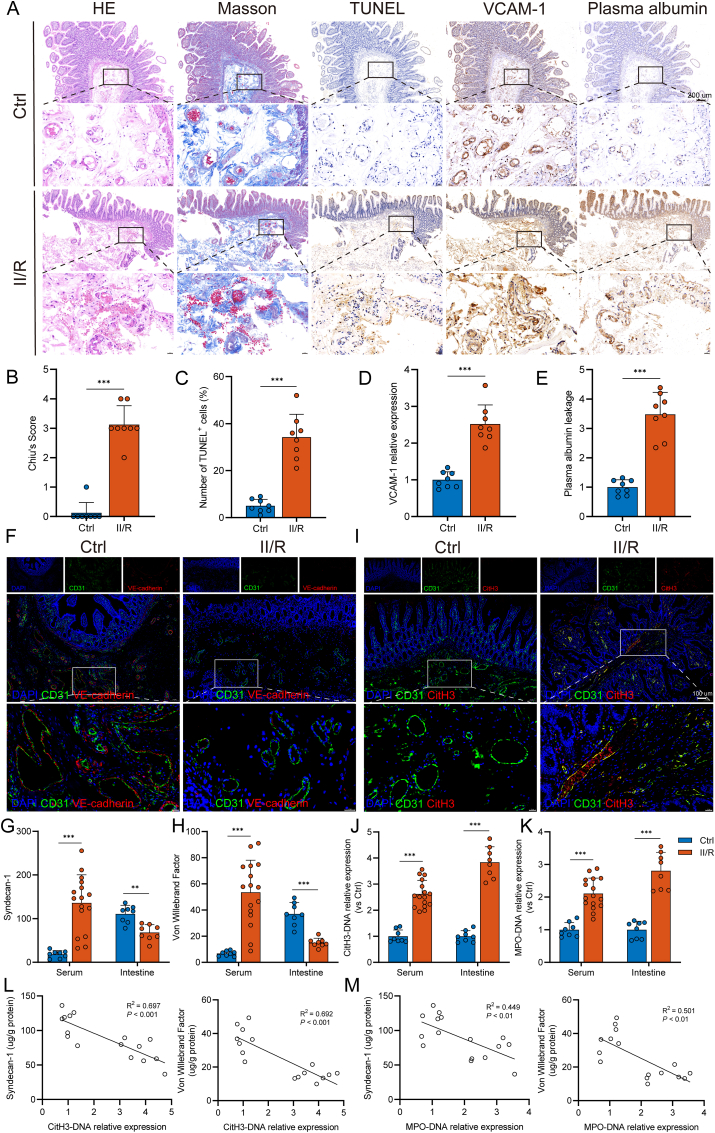
Fig. 1.
Increased NET levels are associated with intestinal microvascular dysfunction in II/R patients. A–E. Representative photographs showing the pathological changes in the intestine from healthy controls and II/R patients, as observed via microscopy. (B) HE- and Masson’s trichrome-stained tissue samples of small bowels were evaluated with the Chiu score system. (C) TUNEL staining was performed to detect apoptosis of intestinal microvascular endothelial cells, and the percentage of TUNEL-positive cells was calculated. (D–E) Immunohistochemical staining for VCAM-1 and albumin was performed in intestinal sections and quantified by the ImageJ software. Scale bars = 200 μm. F. Expression levels of CD31 and VE-cadherin (adherens junction protein of the endothelium) were analyzed by immunofluorescence staining. Scale bars = 100 μm. G. Syndecan-1 levels in the serum (ng/mL) and intestinal tissue lysates (μg/g protein) were evaluated using ELISA kits. H. von Willebrand factor (vWF) concentrations in the serum (ng/mL) and intestinal homogenate (μg/g protein) were evaluated using an ELISA. I. Colocalization of intestinal microvascular (CD31) and NET formation (CitH3) was assessed by immunofluorescence staining. Scale bars = 100 μm. J-K. Circulating and intestinal MPO-DNA and CitH3-DNA complexes in healthy controls and II/R patients were analyzed by ELISAs. L-M. Correlation between intestinal NET biomarkers (CitH3-DNA and MPO-DNA complexes) and intestinal microvascular injury biomarkers (syndecan-1 and vWF). NET, neutrophil extracellular trap; II/R, intestinal ischemia‒reperfusion; HE, hematoxylin-eosin; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling; VCAM-1, vascular cell adhesion protein 1; CitH3, citrullinated histone H3; MPO, myeloperoxidase. Data are shown as the means ± SD, **P < 0.01, ***P < 0.001.