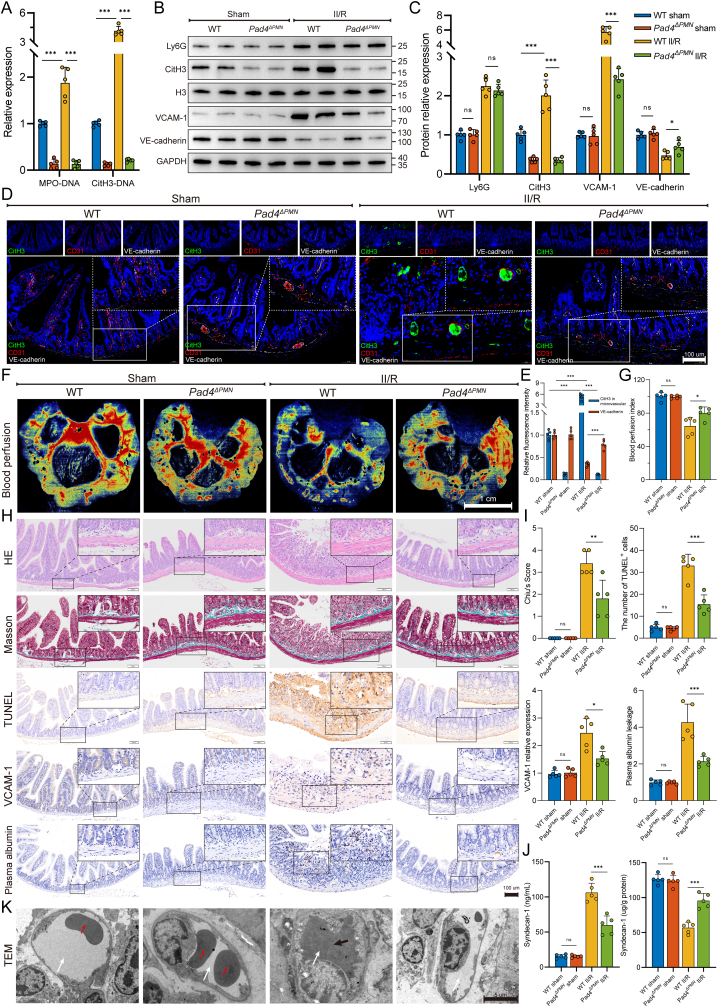
Fig. 2.
Neutrophil-specific Pad4 deficiency attenuated intestinal endothelial damage and microvascular dysfunction. A. Intestinal NET biomarkers, CitH3-DNA and MPO-DNA complexes, were measured by ELISAs. B–C. Protein expression levels of neutrophil infiltration (Ly6G), NET formation (CitH3) and microvascular damage (VCAM-1 and VE-cadherin) biomarkers were measured by western blotting. The grayscale value of each band was analyzed using the ImageJ software. D-E. Immunofluorescent colocalization was performed to assess NET formation (CitH3) in microvascular (CD31) and endothelial (VE-cadherin) injury. Scale bars = 100 μm. F-G. Intestinal blood flow and perfusion indices were measured using a laser speckle blood flow analysis system. Scale bars = 1 cm. H–I. Intestinal tissue of mice was collected for pathological analysis and immunohistochemical staining. The Chiu score system was employed to evaluate HE- and Masson’s trichrome-stained tissue samples from the intestine. To detect apoptosis of intestinal microvascular endothelial cells, TUNEL staining was conducted, and the percentage of TUNEL-positive cells was calculated. Additionally, immunohistochemical staining of VCAM-1 and albumin in intestinal sections was quantified using the ImageJ software. Scale bars = 100 μm. J. Expression of syndecan-1 in the serum and intestinal tissues was examined using ELISA kits. K. Microvascular and endothelial micromorphological changes were observed by transmission electron microscopy (TEM). Following II/R injury in WT mice, irregular swelling of the endothelium and luminal stenosis were observed in intestinal microvessels, along with the presence of microthrombosis. White arrows denoting the lumen, black arrows indicating the presence of microthrombosis, and red arrows highlighting the presence of red blood cells within the lumen. Data are shown as the means ± SD, ns, not significant, *P < 0.05, **P < 0.01, ***P < 0.001.