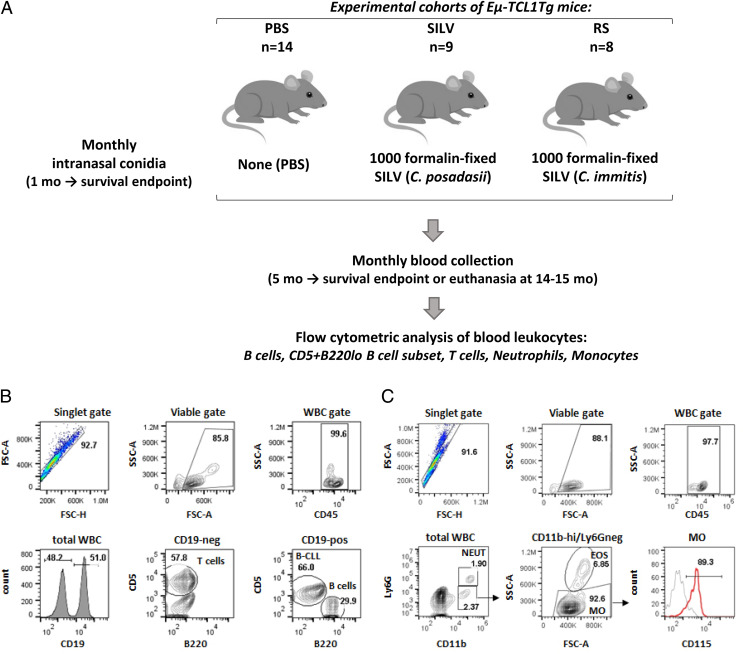
FIGURE 1.
Experimental design and gating strategy for diverse blood leukocytes.
(A) With three cohorts of B-CLL–prone TCL1-Tg mice, we examined whether B-CLL development is affected by repeated inhalation of Coccidioides arthroconidia. The control cohort was exposed to vehicle alone (PBS; n = 14); test cohorts received 1000 fixed C. posadasii conidia (SILV; n = 9) or 1000 fixed C. immitis conidia (RS; n = 8) monthly, beginning at ∼1 mo of age and continuing throughout each recipient’s lifespan. Blood samples were acquired monthly, beginning at 5–6 mo. Total WBCs in individual samples were counted and normal and leukemic cells were distinguished by immunofluorescence staining and flow cytometry. (B) Gating strategy for B and T lymphocytes. Viable singlets were gated for the pan-leukocyte marker, CD45, and assessed for CD19 expression. T cells within the CD19− population were identified by their CD5high status. Normal and leukemic B cells within the CD19+ gate were distinguished as follows: B-CLL cells, CD5+/B220low; normal B cells, CD5−/B220high. (C) Neutrophils and monocytes. Viable singlets, gated for CD45, were evaluated for CD11b and Ly6G expression. Neutrophils are Ly6G+/CD11bhigh; monocyte-enriched WBCs are Ly6G−/CD11bhigh. Contaminating eosinophils were removed from the latter by gating out cells with high side scatter (SSC). The resulting monocyte population (Ly6G−/CD11bhigh/SSClow) expressed CD115, a marker of blood monocytes. Note that in most mice ≤7 mo of age, monocytes were not enumerated due to suboptimal monocyte staining/gating, and neutrophils were gated either as CD45+CD19−CD5−/SSChigh or as CD45+/Ly6G+. Later comparisons indicated that the latter two approaches at neutrophil enumeration yielded results highly concordant with neutrophils gated as CD45+/CD11bhigh/Ly6G+ (C).