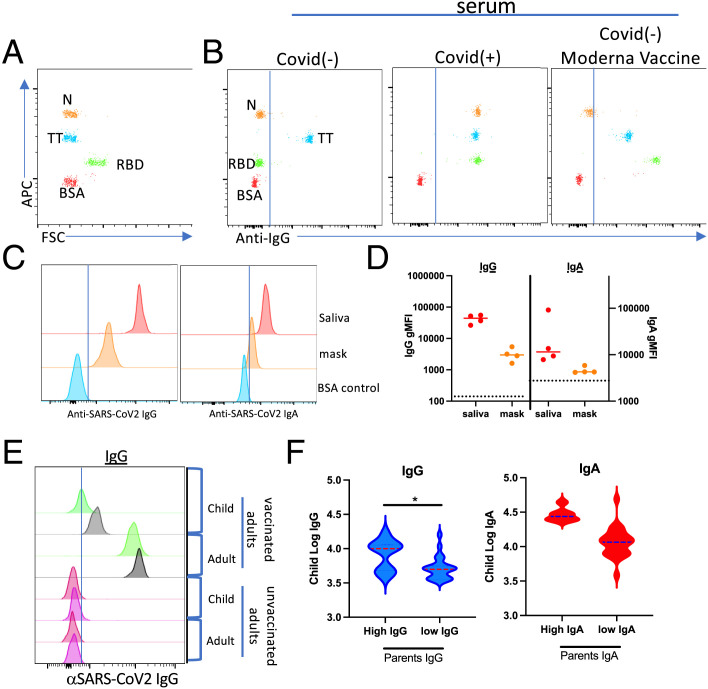
FIGURE 1.
Evidence for aerosol transfer of SARS-CoV-2–specific immunity.
(A and B) Representative flow cytometric results from the use of a multiplex microsphere immunoassay (MMI) (A) evaluating serum samples from a COVID-19–negative (B, left), COVID-19–positive (B, middle), and Moderna mRNA vaccinee (B, right). N, nucleocapsid protein; RBD, receptor-binding domain; TT, tetanus toxoid. Note that the TT reactivity serves as a positive control for validating sample quality in the assay. (C and D) Histograms showing the MFIs for Wuhan-RBD–specific IgG (left) and IgA (right) from saliva or eluted from a surgical mask worn for 1 work day. (D) Quantification of IgG and IgA gMFI eluted from masks obtained from four individuals. Dotted lines indicate gMFI obtained for COVID-19/vaccine− sample. (E) Histograms showing the MFIs for Wuhan-RBD–specific IgG eluted from nasal swabs from unvaccinated children living in households in which parents or family members were either vaccinated (top) or unvaccinated (bottom). Gray and green histograms represent histograms from two separate children in whom high (gray) versus low (green) RBD Abs were identified. (F) Log transformation of the gMFI for Wuhan-RBD–specific IgG (left) or IgA (right) from 34 adult/child pairs using Ab cutoffs for high versus low parental intranasal Ab levels. Cutoff between adult high and low samples was determined as described in Materials and Methods.