FIGURE 2.
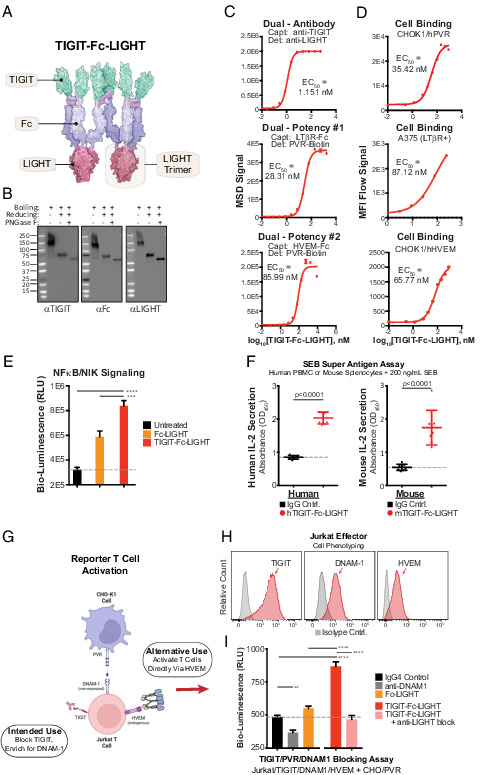
Hexameric TIGIT-Fc-LIGHT engages targets with high affinity and activates effector lymphocytes. (A) TIGIT-Fc-LIGHT was synthesized from a single expression vector in mammalian production cells. Shown is a depiction of the hexamer based on Protein Data Bank structures, with dimerization of the central IgG4 Fc domain and trimerization of the TNF-ligand domain. (B) SDS-PAGE Western blot was used to probe all three domains of TIGIT-Fc-LIGHT under nonreducing, 2-ME reduced, and reduced/deglycosylated (PNGaseF) conditions. (C) Individual MSD binding assays were developed to assess binding to each intended recombinant protein target. (D) Cell-based binding assays were developed to assess cell surface receptor binding (using CHOK1 cells engineered to express hPVR and hHVEM, and also A375 cells that express human LTβR). (E) NF-κB/NIK reporter cells were incubated with a recombinant Fc-LIGHT control or TIGIT-Fc-LIGHT (18 nM each), and signaling activity was assessed through luciferase detection. ***p < 0.001, ****p < 0.0001. (F) TIGIT-Fc-LIGHT (or the murine surrogate, both at 10 nM) activation of T cells through IL-2 induction was assessed in human PBMCs or mouse splenocytes cocultured with the superantigen SEB for 3 d. (G) Schematic of a two-cell reporter system in which PVR-expressing CHO-K1 cells are cocultured with Jurkat effector cells expressing TIGIT and DNAM-1. (H) Jurkat effector cells were phenotyped using flow cytometry for the expression of TIGIT, DNAM-1, and HVEM. (I) Jurkat effector and CHO/hPVR reporter cells were cocultured with (all at 150 nM) an IgG4 control, a DNAM-1–blocking Ab, TIGIT-Fc-LIGHT, or TIGIT-Fc-LIGHT preincubated with a LIGHT-blocking Ab for 6 h, and then luciferase signaling activity was assessed using a luminometer.
