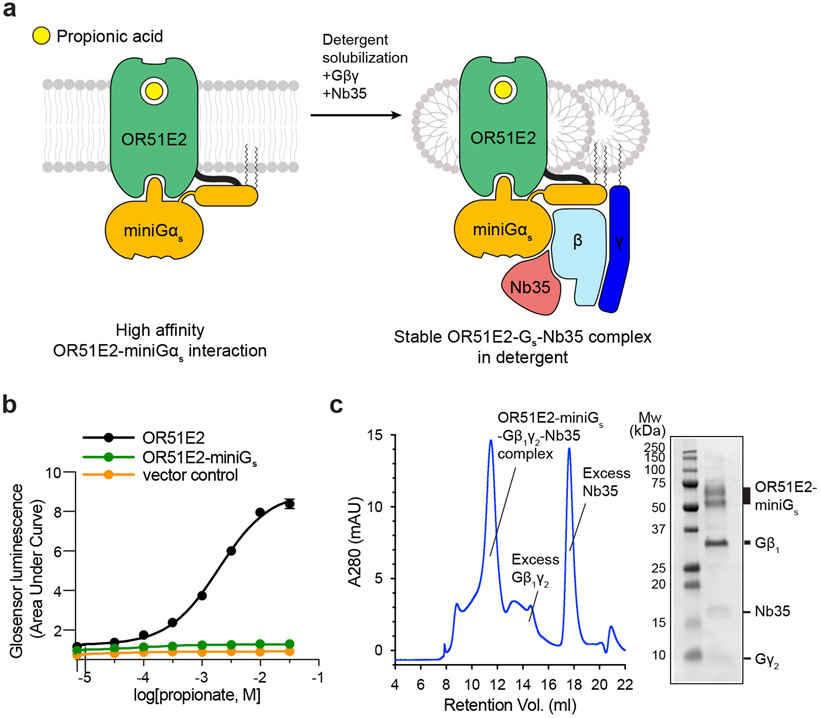
Extended Data Figure 2. Biochemical preparation of OR51E2-Gs complex bound to propionate.
a) Schematic outlining the strategy for stabilization and purification of the activated OR51E2-Gs complex bound to propionate. b) GloSensor cAMP assay demonstrating that fusion of miniGs to OR51E2 blocks activation of endogenous Gs in response to treatment with propionate, suggesting that miniGs couples to the OR51E2 transmembrane core. Data points are the mean of analytical replicates from a representative experiment. Error bars represent the standard deviation between replicates (n=4). c) Size-exclusion chromatogram of purified OR51E2-Gs-Nb35 complex used for structure determinations shown together with a representative SDS-PAGE gel analysis of the collected fraction containing the OR51E2-Gs-Nb35 complex. We observe two bands for OR51E2, likely due to heterogeneous glycosylation of the receptor N-terminus.