Fig 1.
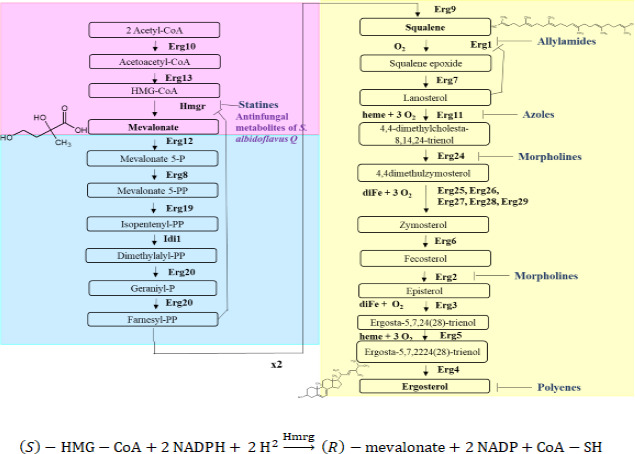
The biosynthetic pathway of ergosterol in yeasts, divided into three modules: the mevalonate pathway (in pink), the farnesyl pyrophosphate pathway (in blue), and the last step leading to ergosterol (in yellow). Enzymes, intermediates, inhibitors, and the requirements for oxygen, heme, and iron are indicated. Inhibitors are listed with their respective target: statins bind with HMGR, allylamines with Erg1, azoles with Erg11, morpholines with Erg2 and Erg24, and polyenes with ergosterol. 3-Hydroxy-3-methyl-glutaryl-CoA reductase (HMGR) is the proposed target for secondary metabolites produced by Streptomyces albidoflavus. Below the boxes, the reaction described is catalyzed by the HMGR enzyme with NADPH as a cofactor.
