ABSTRACT
Aeromonas species are emerging human enteric pathogens. This study examines the isolation of Aeromonas and other enteric bacterial pathogens from patients with and without inflammatory bowel disease (IBD). This study also investigates the intestinal epithelial pathogenic mechanisms of Aeromonas veronii. The isolation rates of seven enteric bacterial pathogens from 2,279 patients with IBD and 373,276 non-IBD patients were compared. An A. veronii strain (AS1) isolated from intestinal biopsies of a patient with IBD was used for pathogenic mechanism investigation, and Escherichia coli K12 was used as a bacterial control. HT-29 cells were used as a model of human intestinal epithelium. A significantly higher isolation of Aeromonas species was found in patients with IBD as compared to non-IBD patients (P = 0.0001, odds ratio = 2.11). A. veronii upregulated 177 inflammatory genes and downregulated 52 protein-coding genes affecting chromatin assembly, multiple small nuclear RNAs, multiple nucleolar RNAs, and 55 cytoplasmic tRNAs in HT-29 cells. These downregulation effects were unique to A. veronii and not observed in HT-29 cells infected with E. coli K12. A. veronii induced intestinal epithelial apoptosis involving the intrinsic pathway. A. veronii caused epithelial microvilli shortening and damage and epithelial production of IL-8. In conclusion, this study for the first time reports the association between IBD and Aeromonas enteric infection detected by bacterial cultivation. This study also reports that A. veronii damages intestinal epithelial cells via multiple mechanisms, of which the downregulating cytoplasmic tRNA, small nuclear RNA, and small nucleolar RNA are novel bacterial pathogenic mechanisms.
IMPORTANCE
This study for the first time reports the association between inflammatory bowel disease (IBD) and Aeromonas enteric infection detected by bacterial pathogen cultivation, highlighting the need of clinical and public health attention. The finding that patients with IBD are more susceptible to Aeromonas enteric infection suggests that detection of Aeromonas enteric infection should be routinely performed for the diagnosis and treatment of IBD. This study also reports novel bacterial pathogenic mechanisms employed by Aeromonas veronii. Through comparative transcriptomic analysis and other techniques, this study revealed the pathogenic mechanisms by which A. veronii causes damage to intestinal epithelial cells. Among the various pathogenic mechanisms identified, the downregulating tRNA, small nuclear and nucleolar RNAs in human intestinal epithelial cells are novel bacterial pathogenic mechanisms.
KEYWORDS: Aeromonas, Aeromonas veronii, transcriptome, IBD, intestinal epithelial cells, Aeromonas caviae, enteric infection, Campylobacter, Shigella, Salmonella
INTRODUCTION
Aeromonas species are facultative anaerobes, gram-negative, rod-shaped bacteria that reside in aquatic environments (1). The Aeromonas genus currently encompasses 36 species, several of which are human pathogens causing gastroenteritis and other diseases such as wound infection and bacteremia (1 – 3).
Inflammatory bowel disease (IBD) is a group of chronic inflammatory conditions of the gastrointestinal tract (4). Crohn’s disease (CD) and ulcerative colitis (UC) are the two major forms of IBD (4). The development of IBD is believed to be triggered by various initiating microorganisms such as Campylobacter species and enteric pathogens, which lead to the breakdown of the tolerance by the mucosal immune system to gut commensal microbes (5, 6).
Previous studies have examined the clinical presentations and treatment of Aeromonas enteric infection in patients with IBD (7 – 10). For example, studies from Lobaton et al. and Guedes et al. reported that patients with IBD infected with Aeromonas were treated more often with antibiotics compared to non-IBD patients (7, 8).
In this study, we examined the prevalence of Aeromonas enteric infection in patients with IBD in comparison with non-IBD patients. This aspect has not been addressed in previous studies, representing a significant gap in knowledge. We also investigated the pathogenic effects and mechanisms on the intestinal epithelial cells using an Aeromonas veronii strain isolated from intestinal biopsies of a patient with IBD via comparative transcriptomic analysis and other experiments. The data from this study provide novel findings regarding Aeromonas enteric infection in patients with IBD. We also present novel pathogenic mechanisms employed by A. veronii in damaging human intestinal epithelial cells.
MATERIALS AND METHODS
Comparison of isolation rates of seven enteric bacterial pathogens from patients with IBD and without IBD
This study utilized data from the isolation of seven enteric bacterial pathogens obtained from 375,842 patients with gastroenteritis symptoms processed at the Douglass Hanly Moir (DHM) Pathology Laboratory Australia between 2015 and 2019. These data were previously analyzed, and the isolation rates of pathogens in different age groups, genders, and seasons were reported (2). In the present study, these data were analyzed to investigate the prevalence of enteric bacterial infections in patients with and without IBD.
For identification of patients with IBD, key words including “IBD,” “inflammatory,” “Crohn,” “ulcerative colitis,” “cd,” and “uc” were used to search the diagnosis recorded for all patients. The obtained initial IBD list was further refined by exclusion of patients with question marks for their IBD diagnosis and IBD family history only. Finally, a total of 375,555 fecal samples, including samples from 2,279 patients with IBD (995 male patients) and 373,276 non-IBD patients (153,295 male patients), were used for comparison of isolations of seven enteric bacterial pathogens including Aeromonas, Campylobacter, Salmonella, Shigella, Plesiomonas, Vibrio, and Yersinia species.
Aeromonas species and other enteric bacterial pathogens analyzed in this study were cultured from fecal samples following the procedures for isolation of enteric pathogens at the DHM, as described previously (2). The same procedures were applied to fecal samples from patients with and without IBD. The presumptive Aeromonas isolates were confirmed by oxidase test and matrix assisted laser desorption ionization/time of flight mass spectrometry (MALDI/TOF-MS) (Vitek MS, Biomerieuz, North Ryde, NSW, Australia), and reported as “Aeromonas species” due to the possibility of misidentifying Aeromonas beyond the genus level by MALDI/TOF-MS.
Examination of the virulence genes in Aeromonas veronii AS1 strain
An Aeromonas veronii strain (AS1) isolated at the DHM from intestinal biopsies of a patient with newly diagnosed CD was available to us. The complete genome of A. veronii AS1 was sequenced in this study to examine the presence of virulence genes. Briefly, the genome of A. veronii AS1 strain was sequenced by Oxford Nanopore and Illumina MiSeq technologies. The details of hybrid assembly and examination of putative virulence factors and secretion systems were described in our previous studies (11, 12). Briefly, for Illumina sequencing, bacterial DNA was extracted using Gentra Puregene Yeast/Bactria Kit (Qiagen), libraries were sequenced on the MiSeq Personal Sequencer, and reads were assembled using Shovill (v 1.0.0). For Nanopore sequencing, bacterial DNA was extracted with phenol-chloroform, libraries were loaded onto FLO-MIN106 flow cell and sequenced on the GridION sequencing device. The complete genome was obtained through hybrid assembly of Illumina and Nanopore reads using Unicycler (v 0.4.7). Putative virulence factors were identified by comparing to known virulence factors in the Virulence Factors Database (13). Secretion systems were examined using MacSyFinder (14).
Comparative transcriptomic analysis of gene expression responses to A. veronii and Escherichia coli strain K12 in human intestinal epithelial cells
A. veronii strain AS1 was used to examine the pathogenic effects and mechanisms of A. veronii infection to human intestinal epithelial cells. The commensal E. coli strain K12 was used as a bacterial control. Human intestinal epithelial cell line HT-29 (ATCC no. HTB-38) was used as a model of human intestinal epithelial cells, which were cultured in McCoy’s 5A medium as previously described (15).
HT-29 cells were cultured in six-well plates (2 × 106/well) for 2 days. Cells were incubated with A. veronii or E. coli K12 in triplicates at multiplicity of infection (MOI) 10 for 4 hours. HT-29 cells without bacterial infection (triplicates) were used as the negative control.
Total RNA extraction, library preparation, and RNA sequencing
Total RNA was isolated from the above bacteria-treated and control HT-29 cells using the Isolate II RNA minikit (Bioline). RNA purity and concentration were measured using a NanoDrop spectrophotometer. The RNA integrity was assessed using TapeStation. Samples with RIN score ≥7 were selected for further analysis.
RNA sequencing (RNA-seq) was conducted at the Ramaciotti Centre for Genomics, University of New South Wales. Briefly, libraries were prepared using Illumina Stranded Total RNA Prep Ligation with Ribo-Zero Plus kit (Illumina) and sequenced on Illumina’s NextSeq 500. At least 60 million 75-bp paired-end reads were generated per sample.
Identification of differentially expressed genes
Gene expressions in A. veronii and E. coli K12-treated HT-29 cells were compared to those in the negative control HT-29 cells. Briefly, sequencing reads were assessed for quality using FastQC (v 0.11.9) and MultiQC (v 0.8). Prior to RNA-seq analysis, Trimmomatic (v 0.40) was used to trim Illumina adapter sequences and filter the raw reads (ILLUMINACLIP, Illumina-PE.fa:2:30:10; LEADING, 3; TRAILING, 3; SLIDINGWINDOW, 4:15; MINLEN, 30) (16). The quality-filtered reads were mapped to the reference human genome GRCh38.p14 using HISAT2 (v 2.1.0). The Subread package Featurecount (v 2.0.1) was used to generate a set of per-gene read counts for each sample using default options (17).
The count data files were loaded into R and analyzed with DESeq2 (v 1.32.0), following standard normalization procedures. Differential gene expressions in HT-29 cells treated with A. veronii or E. coli compared to non-treated cells were calculated as described in other studies (18, 19). The differentially expressed genes were identified using a q-value cut-off of <0.05. Biologically significant differential gene expressions were defined based on q < 0.05 and fold change of 2 or more (log2 fold change ≥ 1 or log2 fold change ≤ −1). The differentially expressed genes were plotted as volcano plots using the EnhancedVolcano package (version 1.14.0) (20).
Gene ontology enrichment analysis
The analysis of differentially expressed genes by gene ontology (GO) enrichment was performed using Enrichr, which provides information on how differentially expressed gene sets impact cellular biological processes. The biological processes involved in responses to A. veronii or E. coli infections in HT-29 cells were identified and ranked by the P value (21).
Quantitative real-time PCR
To verify the RNA-seq data, five significantly differentially expressed RNAs induced by A. veronii and E. coli K12 were randomly selected and subjected to quantitative real-time PCR (qRT-PCR), as previously described (15). These were interleukin 1-beta (IL-1β), DNA damage inducible transcript 4 (DDIT4), MIR210HG, chemokine ligand 20 (CCL20), and interleukin-17C (IL17C). The primers used for qRT-PCR are listed in Table S1.
Staining nucleus and F-actin
HT-29 cells seeded coverslips were incubated for 4 hours with and without A. veronii. Cells were then fixed with 3.6% paraformaldehyde, permeabilized with 0.1% Triton X-100, and blocked using 1% bovine serum albumin. F-actin was then stained using Alexa Fluor 488 Phalloidin (Cell Signaling Technology) following the manufacturer’s instructions. The nuclei were stained using Hoechst 33342. Cells were visualized using Olympus fluorescent microscope BX61.
IL-8 production
The IL-8 production in intestinal epithelial cells induced by A. veronii and E. coli K12 was measured using enzyme-linked immunosorbent assay kit (Invitrogen). Live and heat-killed A. veronii AS1 and E. coli K12 were incubated with HT-29 cells at MOI 1 and 10 as previously described (15).
Caspase 3/7 and caspase 9 activities
HT-29 cells were incubated with A. veronii or E. coli K12 at MOI 1 and 10 for 2 and 4 hours. Levels of active caspase 3/7 and caspase 9 were measured using CellEvent Caspase-3/7 Green ReadyProbes Reagent (Invitrogen) and CaspGLOW Fluorescein Active Caspase-9 Staining Kit (Invitrogen) following the manufacturer’s instructions.
Examining the interaction between A. veronii and intestinal epithelial cells
Gentamicin protection assay
HT-29 cells were infected with A. veronii AS1, E. coli K12 (negative control), E. coli L20 (positive control for adhesion), and Salmonella enterica serovar Typhimurium (positive control for invasion) for 4 hours. Gentamicin protection assay was performed as previously described to examine the adhesion and invasion of A. veronii to HT-29 cells (15).
Scanning and transmission electron microscopy
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were used to further investigate the adhesive and invasive abilities of A. veronii AS1, as well as to visualize morphological changes in HT-29 cells caused by the bacterium. HT-29 cells were infected with A. veronii AS1 at MOI 10 for 2 and 4 hours. SEM and TEM analyses were performed at Mark Wainwright Analytical Centre, Electron Microscope Unit, University of New South Wales.
Briefly, samples were fixed overnight at 4°C using a fixative containing glutaraldehyde in 0.2 M sodium phosphate buffer. Fixed samples were then washed with 0.1 M sodium phosphate buffer followed by dehydration using ethanol. Next, samples were dehydrated using increasing concentrations of hexamethyldisilizane (HMDS) and left to air-dry in a 100% solution of HMDS. Samples were mounted onto SEM stubs, platinum coated, and viewed using an FEI Nova NanoSEM 230 (Oregon) operating at 5 kV.
For TEM, fixed samples were rinsed with 0.1 M sodium phosphate buffer and post-fixed in 1% osmium tetroxide in 0.2 M sodium buffer by using a BioWave Pro+ Microwave Tissue Processor (Ted Pella). After rinsing with 0.1 M sodium phosphate buffer, samples were dehydrated in a graded series of ethanol, infiltrated with resin (Procure, 812), and polymerized using an oven at 60°C for 48 hours. Ultrathin sections (70 nm) were cut using a diamond knife (Diatome) and collected onto carbon-coated copper slot TEM grids. Grids were post-stained using uranyl acetate (2%) and lead citrate. Samples were imaged using a JEOL 1400 TEM operating at 100 kV.
Statistical analysis
The isolation rates of enteric bacterial pathogens in patients with and without IBD were compared by a logistic regression analysis using a binomial generalized linear model in the R Statistical Package (version 4.0.1), with IBD as a categorical independent variable, and the ratio of positive isolation case numbers to the total fecal samples in each patient group, sex, and age as the dependent variables.
For differential gene expression analysis, Wald tests embedded in the DESeq2 package were applied. A one-way analysis of variance (ANOVA) with Dunnett’s test was performed to compare the mRNA levels determined by qRT-PCR, IL-8 concentrations, and caspase activities between samples. P < 0.05 was considered statistically significant.
RESULTS
Aeromonas enteric infection is positively associated with IBD
The isolation rate of Aeromonas species from patients with IBD was 1.18% (27/2279), which was significantly higher than that from non-IBD patients (0.56%, 2105/373,276), with P = 0.0001, the odds ratio (OR) being 2.11 after adjusting for sex (age had no effects in comparison), and 95% confidence interval (CI) of 1.44 to 3.09. The isolation rates of Campylobacter and Salmonella species in IBD patients were significantly lower than those in non-IBD patients (Table 1). The isolation rates of Shigella, Vibrio, and Yersinia species in patients with and without IBD were not statistically different (Table 1).
TABLE 1.
The associations between IBD and enteric infections of Aeromonas species and other six enteric bacterial pathogens a
| Aeromonas | Campylobacter | Salmonella | Plesiomonas | Shigella | Vibrio | Yersinia | |
|---|---|---|---|---|---|---|---|
| IBD | 1.18% (27) | 1.32% (30) | 0.75% (17) | 0.13% (3) | 0 | 0.09% (2) | 0.04% (1) |
| Non-IBD | 0.56% (2,105) | 3.10% (11,553) | 1.39% (5,185) | 0.06% (225) | 0.07% (258) | 0.03% (106) | 0.02% (70) |
| P value | 0.0001 | <0.0001 | 0.009 | 0.18 | 0.908 | 0.119 | 0.406 |
| Odds ratio (95% CI) b | 2.11 (1.44–3.09) | 0.412 (0.287–0.591) | 0.53 (0.328–0.855) | NA c | NA | NA | NA |
A total of 375,555 fecal samples from patients with gastroenteritis symptoms subjected to enteric bacterial pathogen isolation were included for analysis, including samples from 2,279 patients with IBD (average age ± SD: 40.47 ± 19.89, 995 male patients) and 373,276 non-IBD patients (average age ± SD: 40.6 ± 27.55, 153,295 male patients). The percentages for each enteric bacterial pathogen were the positive isolation case numbers (numbers in brackets) divided by fecal samples of each patient group. The isolation rates of enteric bacterial pathogens in patients with and without IBD were compared by a logistic regression analysis using a binomial generalized linear model in the R statistical package (version 4.0.1), with IBD as a categorical independent variable, and the ration of positive isolation case numbers to the total fecal samples in each patient group, sex, and age as the dependent variables. P < 0.05 indicates a statistically significant result.
CI, confidence interval.
NA, not applicable.
A. veronii induced novel gene expression patterns in human intestinal epithelial cells in comparison to E. coli K12
Transcriptomic analysis revealed that both A. veronii and E. coli K12 significantly altered the expression of 454 and 293 genes in HT-29 cells (q < 0.05 and twofold or more changes), respectively. A. veronii infection resulted in downregulation of more transcripts than E. coli K12-infected cells (237 vs 66, respectively) (Fig. 1A and C).
Fig 1.
A. veronii and E. coli K12 upregulated and downregulated expression of genes in human intestinal epithelial HT-29 cells. (A and B) Volcano plot of differentially expressed genes following incubation with A. veronii and E. coli, respectively, for 4 hours as compared with the untreated control cells. The cut-off log2 fold change was ≥1 and ≤ −1, and adjusted P value was <0.05. (B and D) Composition of genes upregulated by A. veronii and E. coli, respectively. (E) Comparison of tRNAs downregulated by A. veronii and E. coli. (F) Fold changes of the 11 mitochondrial tRNAs commonly downregulated by both A. veronii and E. coli. Lines between the data points were colored based on the bacterium that induced the more fold changes. All 11 tRNAs have logarithmic fold change <1 and q < 0.05.
The upregulated transcripts by both A. veronii and E. coli K12 (217 and 227, respectively) were mainly expressed by protein-coding genes involved in inflammation (Fig. 1B and D; Table S2). A. veronii downregulated 52 protein-coding RNAs, 10 of which encoded histone proteins. E. coli downregulated 43 protein-coding RNAs, eight of which coded for heat shock proteins.
A. veronii downregulated 12 small nuclear RNAs, none of which were affected by E. coli K12. A. veronii downregulated 50 small nucleolar RNAs, of which 48 were specific to A. veronii and two were also downregulated by E. coli K12 (Fig. 1B and D; Table S2).
Interestingly, A. veronii downregulated 70 tRNAs, including 55 cytoplasmic tRNAs and 15 mitochondrial tRNAs (Fig. 1E). Eleven of these 15 mitochondrial RNAs were downregulated by both A. veronii and E. coli K12; however, the levels downregulated by E. coli K12 were usually lower (Fig. 1F).
The expression levels of five randomly selected genes, including IL-1β, DDIT4, MIR210HG, CCL20, and IL17C, in HT-29 cells infected with A. veronii or E. coli, and the control HT-29 cells were also tested using qRT-PCR, which showed consistent results with expression patterns revealed using RNA-seq (Fig. S1).
A. veronii downregulated chromatin and nucleosome assembly
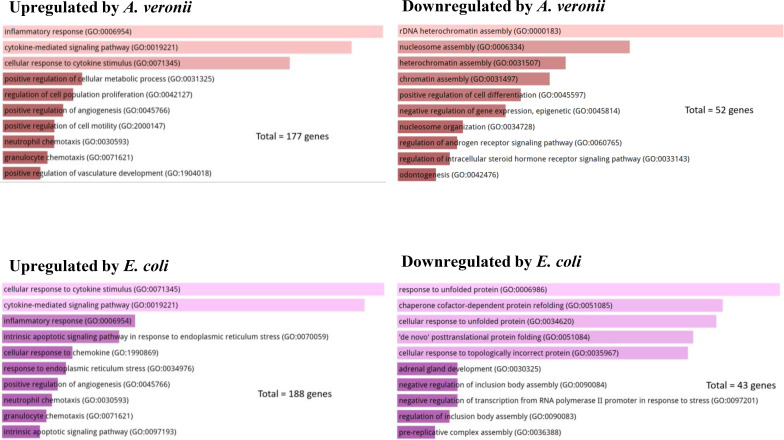
The top 10 (ranked by P value) significantly enriched GO terms in biological process group are displayed in Fig. 2. The upregulated biological processes by A. veronii and E. coli infection were mainly related to inflammation such as inflammatory response and cytokine-mediated signaling pathway (Fig. 2). The striking differences between A. veronii and E. coli in biological impact on cellular physiology in HT-29 cells were from the enrichment of downregulated genes. A. veronii affected chromatin and nucleosome assembly, while E. coli mainly affected protein refolding (Fig. 2).
Fig 2.
The impact of A. veronii and E. coli K12 on biological processes in human intestinal epithelial HT-29 cells. The top 10 enriched gene ontology (GO) terms of protein-coding genes in biological process group are presented, ranked based on P values from lowest to highest. Both A. veronii AS1 and E. coli K12 upregulated biological processes associated with inflammation in HT-29 cells. However, A. veronii AS1 specifically downregulated the top four biological processes related to chromatin assembly, while E. coli K12 downregulated the top five biological processes associated with responses to unfolded proteins in HT-29 cells.
A. veronii damaged the chromatin structure
Nuclear staining of HT-29 cells showed the adverse effect of A. veronii on epithelial cell chromatin structure. In HT-29 cells infected with A. veronii, the chromatins displayed hollow unstained areas which were not observed in the control HT-29 cells (Fig. 3).
Fig 3.
A. veronii caused aberrant chromatin arrangement in human intestinal epithelial HT-29 cells. The fluorescent staining of F-actin and nuclei of HT-29 cells is presented. Compared to HT-29 cells without bacterial infection (untreated), HT-29 cells infected with A. veronii for 4 hours at both MOI 1 and MOI 10 showed an aberrant chromatin arrangement, as indicated by the hollow unstained areas in cell nuclei.
IL-8
Both A. veronii and E. coli K12 induced IL-8 production in HT-29 cells. Following 2-hour incubation, the live A. veronii bacteria and heat-killed A. veronii bacteria at MOI 10 induced a similar level of IL-8 production (908 ± 102 and 937 ± 105 pg/mL, respectively). However, following 4-hour incubation, live A. veronii bacteria at MOI 10 induced IL-8 to be significantly decreased (529 ± 48 and 471 ± 56 pg/mL, respectively) as compared to the IL-8 level of the 2-hour incubation and the heat-killed A. veronii (Fig. 4A and B). Following 4-hour incubation, live E. coli bacteria at MOI 1 and 10 induced IL-8 production to 1,012 ± 76 and 1,010 ± 5 pg/mL, respectively (Fig. 4C).
Fig 4.
A. veronii induced proinflammatory cytokine production, caspase 3/7, and caspase 9 activities in human intestinal epithelial HT-29 cells. (A) Live A. veronii bacteria induced IL-8 production. (B) Heat-killed A. veronii bacteria also induced IL-8 production. The levels of IL-8 are presented after subtraction of IL-8 levels of the untreated control cells. (C) Live E. coli bacteria induced IL-8 production. (D) Live A. veronii bacteria induced significantly higher levels of caspase 3/7 activity. (E) Live E. coli bacteria did not increase caspase 3/7 activity. (F) A. veronii bacteria significantly increased the level of caspase 9 following 4 hours of incubation. A one-way analysis of variance with Dunnett’s test was performed for statistical analysis. Asterisk (*) indicates statistical significance (*P < 0.05, **P < 0.01, and ****P < 0.0001).
A. veronii induced intestinal epithelial cell apoptosis via the intrinsic pathway
A. veronii at both MOI 1 and MOI 10 induced a significantly higher level of caspase 3/7 in HT-29 cells as compared to non-treated HT-29 control cells (P < 0.0001), with fold changes of 16.78 ± 3.32 and 57.39 ± 2.66, respectively (Fig. 4D). A. veronii also induced increased caspase 9 activities (Fig. 4F), indicating the involvement of the intrinsic pathway in A. veronii induced apoptosis. Heat-killed A. veronii bacteria did not induce an increase of caspase 3/7 in HT-29 cells compared to non-treated control HT-29 cells (Fig. 4C). Similarly, live E. coli K12 bacteria did not increase caspase 3/7 in HT-29 cells (Fig. 4E).
A. veronii adhered to intestinal epithelial cells and caused microvilli shortening
A. veronii showed significantly higher levels of adhesion compared to E. coli K12 and E. coli L20 (P < 0.05). Values for adhesion for HT-29 cells infected with A. veronii, E. coli K12, and E. coli L20 were 3.95E7 ± 5.00E6 CFU/mL, 2.88E6 ± 3.43E5 CFU/mL, and 1.68E07 ± 1.17E5 CFU/mL, respectively (Fig. 5A). No A. veronii growth from lysed HT-29 cells after gentamicin treatment was observed, showing no bacterial invasion to HT-29 cells. SEM and TEM both showed adhesion of A. veronii to HT-29 cells (Fig. 5B, C, and D). SEM revealed that A. veronii caused the shortening or disappearance of microvilli, which was observed to initiate at 2 hours and became more pronounced after 4 hours (Fig. 5C and D), and TEM showed membrane blebbing in A. veronii-treated cells (Fig. 5D).
Fig 5.
Adhesion of A. veronii to human intestinal epithelial HT-29 cells. (A) Both A. veronii AS1 and the positive control E. coli L20 showed a significantly higher level of adhesion to HT-29 cells as compared to that of the negative control E. coli K12 after 4 hours of incubation. (B) TEM shows the attachment of A. veronii to HT-29 cells after 4 hours of incubation. (C and D) SEM shows A. veronii caused microvilli shortening or disappearance, as indicated by white arrows. A one-way analysis of variance with Dunnett’s test was performed for statistical analysis. Asterisk (*) indicates statistical significance (**P < 0.01, ***P < 0.001, and ****P < 0.0001).
A. veronii strain AS1 genome and virulence factors
The complete genome of A. veronii strain AS1 was successfully sequenced. Two hundred and forty-six putative virulence genes were identified in A. veronii AS1. Genes encoding secreted toxins such as aerolysin, hemolysins, microbial collagenase, and StcE (secreted protease of C1-esterase inhibitor) were present (Fig. 6). T1SS and T2SS were found in the genome of A. veronii strain AS1. T3SS, T4SS, and T6SS were absent.
Fig 6.
Putative virulence factors in Aeromonas veronii strain AS1. A. veronii strain AS1 was isolated from intestinal biopsies of a patient with newly diagnosed Crohn’s disease. The complete genome of A. veronii strain AS1 was sequenced in this study, and putative virulence factors were identified through searches of the Virulence Factors Database. A total of 246 virulence genes were identified in A. veronii AS1 genome. Genes encoding secreted toxins such as aerolysin, hemolysins, microbial collagenase, and StcE (secreted protease of C1-esterase inhibitor) were identified. T1SS and T2SS secretion systems were found in the genome of A. veronii strain AS1. However, T3SS, T4SS, and T6SS secretion systems were found to be absent.
DISCUSSION
Aeromonas species are emerging human enteric pathogens. However, much remains unknown about the prevalence of Aeromonas enteric infection in patients with IBD and the Aeromonas enteric pathogenic mechanisms. In this study, we investigated the enteric infection rates of Aeromonas and other six enteric bacterial pathogens in patients with and without IBD and examined the intestinal epithelial pathogenic effects and mechanisms induced by A. veronii via comparative transcriptomic analysis. Our study discovered an association between the prevalence of Aeromonas enteric infection and IBD, as well as novel bacterial pathogenic mechanisms.
Through comparison of the bacterial culture positivity of Aeromonas and other six enteric pathogens in patients with and without IBD who presented with gastroenteritis symptoms, we found a positive association between IBD and enteric infection of Aeromonas species detected by bacterial culture from fecal samples (OR = 2.11), showing that patients with IBD are more susceptible to Aeromonas enteric infections (Table 1). We also found a significantly lower infection rate of Campylobacter and Salmonella in patients with IBD as compared to non-IBD patients. However, the reason why patients with IBD had a higher Aeromonas enteric infection, but a lower Campylobacter and Salmonella infection is not clear.
Our study did not have the capacity to determine whether Aeromonas enteric infection plays a role in the pathogenesis of IBD. However, previous studies suggest that Aeromonas enteric infection may play a role in triggering the development of IBD or relapse in some patients (7, 9, 10). For example, Lobaton et al. conducted a study in Belgium, involving 11 IBD patients and 66 non-IBD patients, and found that Aeromonas enteric infection triggered a moderate to severe flare in two patients with UC (7). They also reported the isolation of Aeromonas species from two patients with newly diagnosed CD (4). Another study by Willoughby et al. from the UK reported that three patients progressed to UC after Aeromonas hydrophila or Aeromonas sobria infection (9). Despite the interesting findings, these earlier studies had limited sample sizes. Therefore, it is crucial to conduct future longitudinal prospective studies involving larger cohorts of patients to investigate the potential role of Aeromonas enteric infection in the pathogenesis of IBD.
Currently, the routine screening for enteric bacterial enteric pathogens in many diagnostic laboratories does not include Aeromonas species. Our finding that patients with IBD are more prone to Aeromonas enteric infection highlights the importance of screening for Aeromonas species in patients with IBD presenting with gastrointestinal symptoms. By implementing such screening measures, targeted antibiotic intervention can be promptly provided. This viewpoint is further supported by previous clinical studies, which showed that patients with IBD infected with Aeromonas experienced more complications and required more frequent antibiotic treatment as compared to non-IBD patients (7, 8).
Several Aeromonas species such as A. veronii, Aeromonas caviae, and A. hydrophila have been isolated from patients with and without IBD (1 – 4). Lobaton et al. reported that A. veronii more frequently caused severe infection than the other species (7). In the DHM diagnostic laboratory, Aeromonas isolates were reported as “Aeromonas species” due to the potential for misidentification beyond the genus level by MALDI/TOF-MS. In our study, we had access to an Aeromonas isolate cultured from intestinal biopsies of a patient with newly diagnosed CD. The complete genome sequencing of this isolate revealed it to be A. veronii (AS1 strain), which was further used for transcriptomic analyses and examination of virulence genes. We identified over 200 virulence genes within the genome of A. veronii AS1 strain (Fig. 6). Some of these virulence factors have been characterized in other bacteria. For example, aerolysin in A. hydrophila acts as a pore-forming toxin (22, 23). StcE metalloprotease was initially found in enterohaemorrhagic E. coli O157:H7, which cleaves C1 inhibitor and mucins (24). In a previous study, we examined the genomes of 168 A. veronii strains isolated from various sources, including patients with gastroenteritis, and found that 106 A. veronii strains (63%) possessed T3SS secretion systems (7). T3SS secretion systems enable bacterial pathogens to inject bacterial effector proteins directly into host cell cytoplasm (25). In our previous study, we found that 48% A. veronii strains isolated from patients with gastrointestinal infections possessed T3SS system (7). However, the AS1 strain sequenced in this study lacks the T3SS system. Future studies should aim to examine the genomes of Aeromonas strains isolated from patients with IBD and other human diseases to identify genomic features that may be potentially associated with different diseases.
We employed comparative transcriptomic analysis to identify the biological pathways that are disturbed in human intestinal epithelial cells by A. veronii, a leading Aeromonas enteric pathogen. E. coli K12 strain, a commensal gut bacterium with some properties common to A. veronii such as being a facultative anaerobic motile rod, was used as the control bacterial species. RNAs upregulated by A. veronii were mainly from protein-coding genes related to inflammation such as CCL20, IL-8, and IL-17C. Many of these molecules were also upregulated by E. coli K12, showing that they were the common epithelial responses to bacteria (Fig. 1; Table S2). However, IL-1β RNA was upregulated only by A. veronii, suggesting that some specific responses were triggered toward A. veronii.
The RNAs downregulated by A. veronii and E. coli K12 presented a markedly different picture. Nearly 20% of protein-coding RNAs downregulated by A. veronii were expressed by genes coding for histones, which were not seen in E. coli K12 treated HT-29 cells (Table S2). The histone proteins are for packaging DNA into nucleosomes, then condensing them into chromatin (26, 27). The biological process of nucleosome and chromatin assemblies in intestinal epithelial cells was clearly affected by A. veronii (Fig. 2). When the nuclei of intestinal epithelial HT-29 cells were stained, it was revealed that the chromatin structure of HT-29 cells was indeed damaged by A. veronii (Fig. 3). Chromatin is essential for fundamental cell processes such as DNA replication, transcription, and cell division (28). A. veronii also specifically downregulated 12 small nuclear RNAs and 48 nucleolar RNAs. These RNAs are for splicing of introns from pre-mRNA and maturation of ribosomal RNAs, respectively (29 – 31). A. veronii also downregulated large numbers of tRNAs which are critical for protein synthesis (32, 33). Overall, A. veronii downregulated the cellular processes of intestinal epithelial cells at both transcriptional and translational levels. One direct consequence is that such a downregulation may adversely affect the production of epithelial cytokines, resulting in compromised immune responses. Indeed, we showed that live A. veronii bacteria compromised the production of IL-8 as compared to dead A. veronii bacteria and live E. coli K12 (Fig. 4).
tRNAs are also known to inhibit apoptosis by blocking the activation of caspase 9 through binding to cytochrome c, which impairs the formation of apoptosome (34). Degradation of tRNA using RNA hydrolysis was previously demonstrated to enhance apoptosis via the intrinsic pathway (34). A. veronii induced apoptosis in HT-29 cells as indicated by the increased caspase 3/7 activities, and the apoptosis was involved in the intrinsic pathway as indicated by increased caspase 9 activities (Fig. 4). These data strongly support the role of tRNA downregulation by A. veronii in contributing to intestinal epithelial cell apoptosis. A veronii at MOI 10 induced a higher level of caspase 3/7 in HT-29 cells than MOI 1 (Fig. 4), suggesting that patients infected with a higher load of A. veronii may develop more severe clinical symptoms. Heat-killed A. veronii induced IL-8 production in HT-29 cells but not apoptosis, showing that the virulence factors responsible for epithelial cell death were heat labile (Fig. 4).
A. veronii adhered to intestinal epithelial cells, reduced epithelial microvilli, and caused membrane blebbing (Fig. 5). Previous studies have shown that reorganization of cytoskeletal structures drives the formation of membrane blebs and apoptotic bodies and accompanying the formation of membrane blebbing is the loss of cell surface microvilli (35 – 38).
In summary, we for the first time report an association between the prevalence of Aeromonas enteric infection and IBD using bacterial isolation from fecal samples. Furthermore, we show that A. veronii damages intestinal epithelial cells through multiple mechanisms (Fig. 7), of which the downregulating cytoplasmic tRNA, small nuclear RNA, and small nucleolar RNA are novel bacterial pathogenic mechanisms.
Fig 7.
Summary of the pathogenic effects and mechanisms by which A. veronii bacteria damage human intestinal epithelial cells. The A. veronii AS1 strain induces various detrimental effects in human intestinal epithelial HT-29 cells. These effects include the upregulation of multiple proinflammatory molecules such as IL-1β and IL-8, the downregulation of gene expression of tRNAs, small nuclear RNAs, and small nucleolar RNAs, the downregulation of expression of genes encoding histone proteins, the induction of epithelial cell apoptosis through the intrinsic pathway by increasing caspase 3/7 and caspase 9 activities, the adherence to intestinal epithelial cells, the promotion of microvillous atrophy, and the disruption of chromatin arrangement leading to aberrant patterns.
ACKNOWLEDGMENTS
We would like to thank Dr. Eve Slavich, a statistical consultant at the Stats Central, Mark Wainwright Analytical Centre, University of New South Wales, for her advice on statistical analysis of the isolation rates of enteric bacterial pathogens in patients with and without IBD. Seul A. Lee and Christopher Yuwono would like to thank the support from the Australian Government Research Training Program Scholarship.
This project was supported by the Boost Award and the Faculty Research Grant (grant no. PS46772) awarded to Associate Professor Li Zhang from the University of New South Wales. The cost for RNA sequencing of E. coli K12 infected HT-29 cells was supported by Professor Ruiting Lan.
Seul A. Lee conducted most of the experiments. Fang Liu conducted caspase 9 experiment and also conducted F-actin and nuclear staining and caspase 3/7 together with Seul A. Lee. Christopher Yuwono conducted the analysis of enteric bacterial pathogen isolations in patients with IBD and gastroenteritis. Monique Phan conducted transcriptomic analysis and qRT-PCR for E. coli K12-treated cells and controls. Sarah Chong together with Fang Liu conducted the measurements of caspase 9. Joanna M. Biazik conducted electron microscopic analysis. Alfred Chin Yen Tay conducted genome sequencing for Aeromonas strains. Michael C. Wehrhahn organized data of Aeromonas isolation and provided Aeromonas strains. Michael Janitz provided expert guidance on initiating transcriptomic study. Stephen Riordan conceived the project together with Li Zhang and provided critical feedback on IBD. Ruiting Lan conceived the RNA sequencing for E. coli K12-treated HT-29 cells together with Li Zhang. Li Zhang conceived, organized, and supervised the project. Li Zhang and Fang Liu played a major role in manuscript writing. All authors provided critical feedback and helped in editing the manuscript.
The authors declare no competing interests.
Contributor Information
Ruiting Lan, Email: r.lan@unsw.edu.au.
Michael C. Wehrhahn, Email: mwehrhahn@dhm.com.au.
Li Zhang, Email: l.zhang@unsw.edu.au.
Shannon D. Manning, Michigan State University, East Lansing, Michigan, USA
ETHICS APPROVAL
The use of bacterial isolation data provided by DHM for a retrospective study, which did not involve patient consent, has been approved by the University of New South Wales HREAP Executive (HC200755).
DATA AVAILABILITY
Complete genome of A. veronii AS1 was submitted to NCBI bacterial genome database under accession number CP114182. The RNA-seq data have been submitted to NCBI Gene Expression Omnibus under accession GSE222117.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.01088-23.
Supplementary Materials including Supplementary Figure 1, Supplementary Table 1 and Supplementary Table 2.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Fernández-Bravo A, Figueras MJ. 2020. An update on the genus Aeromonas: taxonomy, epidemiology, and pathogenicity. Microorganisms 8:129. doi: 10.3390/microorganisms8010129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yuwono C, Wehrhahn MC, Liu F, Riordan SM, Zhang L. 2021. The isolation of Aeromonas species and other common enteric bacterial pathogens from patients with gastroenteritis in an Australian population. Microorganisms 9:1440. doi: 10.3390/microorganisms9071440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Teunis P, Figueras MJ. 2016. Reassessment of the enteropathogenicity of mesophilic Aeromonas species. Front Microbiol 7:1395. doi: 10.3389/fmicb.2016.01395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Halfvarson J, Brislawn CJ, Lamendella R, Vázquez-Baeza Y, Walters WA, Bramer LM, D’Amato M, Bonfiglio F, McDonald D, Gonzalez A, McClure EE, Dunklebarger MF, Knight R, Jansson JK. 2017. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat Microbiol 2:17004. doi: 10.1038/nmicrobiol.2017.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang L, Liu F, Xue J, Lee SA, Liu L, Riordan SM. 2022. Bacterial species associated with human inflammatory bowel disease and their pathogenic mechanisms. Front Microbiol 13:801892. doi: 10.3389/fmicb.2022.801892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Azimi T, Nasiri MJ, Chirani AS, Pouriran R, Dabiri H. 2018. The role of bacteria in the inflammatory bowel disease development: a narrative review. APMIS 126:275–283. doi: 10.1111/apm.12814 [DOI] [PubMed] [Google Scholar]
- 7. Lobatón T, Hoffman I, Vermeire S, Ferrante M, Verhaegen J, Van Assche G. 2015. Aeromonas species: an opportunistic enteropathogen in patients with inflammatory bowel diseases? a single center cohort study. Inflamm Bowel Dis 21:71–78. doi: 10.1097/MIB.0000000000000247 [DOI] [PubMed] [Google Scholar]
- 8. Pereira Guedes T, Alves Silva J, Neves S, Falcão D, Costa P, Lago P, Pedroto I, Salgado M. 2023. Positioning Aeromonas infection in inflammatory bowel disease: a retrospective analysis. GE Port J Gastroenterol 30:20–28. doi: 10.1159/000520272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Willoughby JM, Rahman AF, Gregory MM. 1989. Chronic colitis after Aeromonas infection. Gut 30:686–690. doi: 10.1136/gut.30.5.686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahishali E, Pinarbasi B, Akyuz F, Ibrisim D, Kaymakoglu S, Mungan Z. 2007. A case of Aeromonas hydrophila enteritis in the course of ulcerative colitis. Eur J Intern Med 18:430–431. doi: 10.1016/j.ejim.2006.12.008 [DOI] [PubMed] [Google Scholar]
- 11. Liu F, Yuwono C, Tay ACY, Wehrhahn MC, Riordan SM, Zhang L. 2022. Analysis of global Aeromonas veronii genomes provides novel information on source of infection and virulence in human gastrointestinal diseases. BMC Genomics 23:166. doi: 10.1186/s12864-022-08402-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu F, Chen S, Luu LDW, Lee SA, Tay ACY, Wu R, Riordan SM, Lan R, Liu L, Zhang L. 2020. Analysis of complete Campylobacter concisus genomes identifies genomospecies features, secretion systems and novel plasmids and their association with severe ulcerative colitis. Microb Genom 6:mgen000457. doi: 10.1099/mgen.0.000457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen L, Yang J, Yu J, Yao Z, Sun L, Shen Y, Jin Q. 2005. VFDB: a reference database for bacterial virulence factors. Nucleic Acids Res 33:D325–8. doi: 10.1093/nar/gki008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abby SS, Néron B, Ménager H, Touchon M, Rocha EPC. 2014. MacSyFinder: a program to mine genomes for molecular systems with an application to CRISPR-Cas systems. PLoS One 9:e110726. doi: 10.1371/journal.pone.0110726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee SA, Wang Y, Liu F, Riordan SM, Liu L, Zhang L. 2020. Escherichia coli K12 upregulates PD-L1 expression in IFN-γ sensitized intestinal epithelial cells via the NF-κB pathway. Infect Immun 89:e00618-20. doi: 10.1128/IAI.00618-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liao Y, Smyth GK, Shi W. 2014. featurecounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30:923–930. doi: 10.1093/bioinformatics/btt656 [DOI] [PubMed] [Google Scholar]
- 18. Love MI, Huber W, Anders S. 2014. Differential analysis of count data–the DESeq2 package. Genome Biol 15:10–1186. doi: 10.1186/s13059-014-0550-8 [DOI] [Google Scholar]
- 19. Robinson MD, McCarthy DJ, Smyth GK. 2010. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26:139–140. doi: 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blighe K, Rana S, Lewis M. EnhancedVolcano: publication-ready volcano plots with enhanced colouring and labeling. R package version 1.14.0. Available from: https://github.com/kevinblighe/EnhancedVolcano
- 21. Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, Koplev S, Jenkins SL, Jagodnik KM, Lachmann A, McDermott MG, Monteiro CD, Gundersen GW, Ma’ayan A. 2016. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res 44:W90–7. doi: 10.1093/nar/gkw377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bücker R, Krug SM, Rosenthal R, Günzel D, Fromm A, Zeitz M, Chakraborty T, Fromm M, Epple H-J, Schulzke J-D. 2011. Aerolysin from Aeromonas hydrophila perturbs tight junction integrity and cell lesion repair in intestinal epithelial HT-29/B6 cells. J Infect Dis 204:1283–1292. doi: 10.1093/infdis/jir504 [DOI] [PubMed] [Google Scholar]
- 23. Dal Peraro M, van der Goot FG. 2016. Pore-forming toxins: ancient, but never really out of fashion. Nat Rev Microbiol 14:77–92. doi: 10.1038/nrmicro.2015.3 [DOI] [PubMed] [Google Scholar]
- 24. Grys TE, Walters LL, Welch RA. 2006. Characterization of the StcE protease activity of Escherichia coli O157: H7. J Bacteriol 188:4646–4653. doi: 10.1128/JB.01806-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deng W, Marshall NC, Rowland JL, McCoy JM, Worrall LJ, Santos AS, Strynadka NCJ, Finlay BB. 2017. Assembly, structure, function and regulation of type III secretion systems. Nat Rev Microbiol 15:323–337. doi: 10.1038/nrmicro.2017.20 [DOI] [PubMed] [Google Scholar]
- 26. Luger K, Dechassa ML, Tremethick DJ. 2012. New insights into nucleosome and chromatin structure: an ordered state or a disordered affair? Nat Rev Mol Cell Biol 13:436–447. doi: 10.1038/nrm3382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fyodorov DV, Zhou B-R, Skoultchi AI, Bai Y. 2018. Emerging roles of linker histones in regulating chromatin structure and function. Nat Rev Mol Cell Biol 19:192–206. doi: 10.1038/nrm.2017.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Papamichos-Chronakis M, Peterson CL. 2013. Chromatin and the genome integrity network. Nat Rev Genet 14:62–75. doi: 10.1038/nrg3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fica SM, Tuttle N, Novak T, Li N-S, Lu J, Koodathingal P, Dai Q, Staley JP, Piccirilli JA. 2013. RNA catalyses nuclear pre-mRNA splicing. Nature 503:229–234. doi: 10.1038/nature12734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kufel J, Grzechnik P. 2019. Small nucleolar RNAs tell a different tale. Trends Genet 35:104–117. doi: 10.1016/j.tig.2018.11.005 [DOI] [PubMed] [Google Scholar]
- 31. Henras AK, Plisson-Chastang C, O’Donohue M-F, Chakraborty A, Gleizes P-E. 2015. An overview of pre‐ribosomal RNA processing in eukaryotes. Wiley Interdiscip Rev RNA 6:225–242. doi: 10.1002/wrna.1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schimmel P. 2018. The emerging complexity of the tRNA world: mammalian tRNAs beyond protein synthesis. Nat Rev Mol Cell Biol 19:45–58. doi: 10.1038/nrm.2017.77 [DOI] [PubMed] [Google Scholar]
- 33. Torrent M, Chalancon G, de Groot NS, Wuster A, Madan Babu M. 2018. Cells alter their tRNA abundance to selectively regulate protein synthesis during stress conditions. Sci Signal 11:eaat6409. doi: 10.1126/scisignal.aat6409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mei Y, Yong J, Liu H, Shi Y, Meinkoth J, Dreyfuss G, Yang X. 2010. tRNA binds to cytochrome C and inhibits caspase activation. Mol Cell 37:668–678. doi: 10.1016/j.molcel.2010.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mills JC, Stone NL, Erhardt J, Pittman RN. 1998. Apoptotic membrane blebbing is regulated by myosin light chain phosphorylation. J Cell Biol 140:627–636. doi: 10.1083/jcb.140.3.627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cotter TG, Lennon SV, Glynn JM, Green DR. 1992. Microfilament-disrupting agents prevent the formation of apoptotic bodies in tumor cells undergoing apoptosis. Cancer Res. 52:997–1005. [PubMed] [Google Scholar]
- 37. Torgerson RR, McNiven MA. 1998. The actin-myosin cytoskeleton mediates reversible agonist-induced membrane blebbing. J Cell Sci 111:2911–2922. doi: 10.1242/jcs.111.19.2911 [DOI] [PubMed] [Google Scholar]
- 38. Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. 2001. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol 3:339–345. doi: 10.1038/35070009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials including Supplementary Figure 1, Supplementary Table 1 and Supplementary Table 2.
Data Availability Statement
Complete genome of A. veronii AS1 was submitted to NCBI bacterial genome database under accession number CP114182. The RNA-seq data have been submitted to NCBI Gene Expression Omnibus under accession GSE222117.