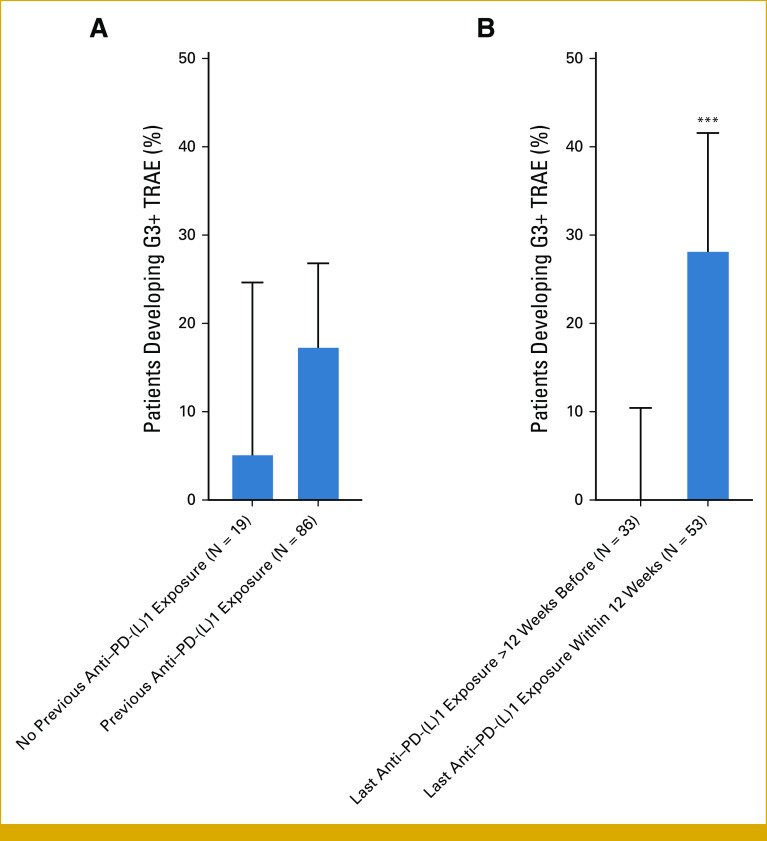
FIG 3.
Influence of previous anti–PD-(L)1 therapy exposure on incidence of severe sotorasib-related adverse events. (A) Incidence of G3+ sotorasib-related adverse events among patients with no previous anti–PD-(L)1 exposure (1/19; 5%) and previous exposure (15/86; 17%). (B) Among patients with previous anti–PD-(L)1 exposure (N = 86 total), incidence of G3+ sotorasib-related TRAEs among patients with last anti–PD-(L)1 exposure more than 12 weeks before initiation of sotorasib (0/33; 0%) and among patients with last exposure within 12 weeks of initiation of sotorasib (15/53; 28%). ***P < .001 for Fisher's exact test. G3+, grade 3 or higher; TRAE, treatment-related adverse event.