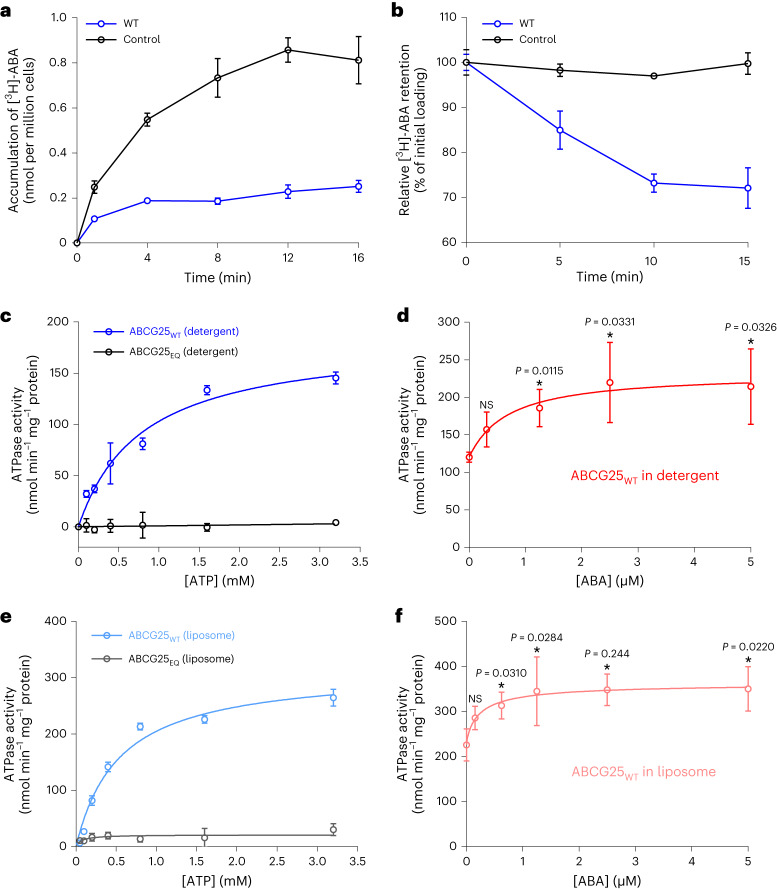
Fig. 1. Characterization of ABCG25-mediated ABA transport and ATPase activity.
a, Loading assay showing that accumulation of [3H]-ABA is decreased in cells expressing ABCG25 compared with untransfected control cells. All data points represent five independent measurements. Data are mean ± s.d. b, Efflux assay showing that [3H]-ABA retention is decreased in cells expressing ABCG25 compared with control cells. Data points represent three independent measurements. Data are mean ± s.d. c, ATPase activities of ABCG25WT and ABCG25EQ in detergent micelle in the presence of different concentrations of ATP. Data points were nonlinear-fitted using the Michaelis–Menten equation. Data points represent four independent measurements. Data are mean ± s.d. d, ABA concentration-dependent ATPase activity of ABCG25WT in detergent micelle. Data points were nonlinear-fitted using the allosteric sigmoidal model. Data points represent three independent measurements. NS, not significant; *P < 0.05 for ABA-added versus no ABA (one-way analysis of variance (ANOVA) with Dunnett’s multiple comparisons test). Data are mean ± s.d. e, ATPase activities of ABCG25WT and ABCG25EQ reconstituted into liposomes in the presence of different concentrations of ATP. Data points were nonlinear-fitted using the Michaelis–Menten equation. Data points represent four independent measurements. Data are mean ± s.d. f, ABA concentration-dependent ATPase activity of ABCG25WT reconstituted into liposomes. Data points were nonlinear-fitted using the allosteric sigmoidal model. Data points represent three independent measurements. *P < 0.05 for ABA-added versus no ABA (one-way ANOVA with Dunnett’s multiple comparisons test). Data are mean ± s.d.