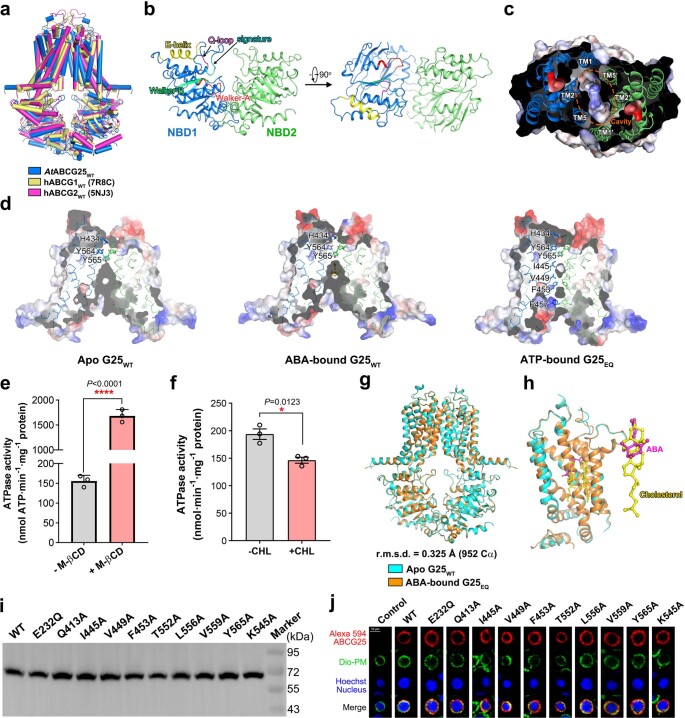
Extended Data Fig. 5. Structure analysis of ABCG25.
a, Structure alignments of ABCG25WT and hABCG1WT (PDB code: 7R8C), hABCG2WT (PDB code: 5NJ3) in the apo-state. b, The separated NBDs of ABCG25WT in the apo-state. The conserved motifs such as walker A, Q-loop, ABC signature, walker B, and E-helix are indicated. c, The substrate-binding cavity formed by TM1, TM2 and TM5a from the opposing monomers. d, Section view of the surface electrostatic potential of ABCG25 TMDs in the apo-, ABA-bound or ATP-bound state. The residues at the exit of transport cavity and along the substrate translocation pathway are labeled aside and shown as sticks. e, ATPase activities of ABCG25WT in detergent micelle in the absence or presence of 0.5mM M-βCD. Three independent experiments were performed for each construct (n = 3). **** P < 0.0001 (two-tailed unpaired t-test). Data are mean ± SEM. f, ATPase activities of ABCG25WT in liposome reconstituted with or without cholesterol (lipid:cholesterol prepared at a 9:1(w/w) ratio). Three independent experiments were performed for each construct (n = 3). * P = 0.0123 (two-tailed unpaired t-test). Data are mean ± SEM. g, Structure alignments of ABCG25WT in the apo- and ABA-bound state. The ABA-bound structure (coloured orange) is almost identical to the apo-state structure (coloured cyan). h, Zoom-in views of the substrate-binding site of ABCG25 in the apo- and ABA-bound state and superposition of ABA and cholesterol. i, Western blotting analysis of the expression level of ABCG25 variants using anti-Flag antibody. Experiments have been repeated at least twice with similar results. Uncropped blot can be found in Source Data. j, Confocal microscopy representative images illustrating the localization of ABCG25 variants. Empty or ABCG25-expressing Sf9 cells were immunostained with anti-Flag Alexa Fluor 594-conjugated antibody and counterstained with Dio and Hoechst 33342. All the variants have an N-terminal Flag tag. Red, Alexa 594, ABCG25; green, Dio, plasma membrane; blue, Hoechst 33342, cell nucleus; merge, overlay among Alexa 594, Dio and Hoechst 33342. Scale bar = 10 μm. Experiments have been repeated at least twice with similar results.