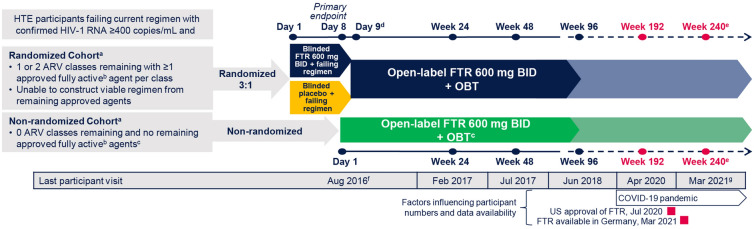
Fig. 1.
Study design. ARV antiretroviral, BID twice daily, FTR fostemsavir, HTE heavily treatment-experienced, OBT optimized background therapy. aThere were no screening temsavir susceptibility criteria. bFully active is based on susceptibility (current or historical resistance measures) and availability [the participant is tolerant of, eligible for, and willing to take (in the case of enfuvirtide only) the ARV]. cUse of investigational agents as part of OBT was permitted in the non-randomized cohort only. dSubsequent time points were measured from the start of open-label FTR 600 mg BID + OBT. eThe study is expected to be conducted until participants can access FTR through other means (e.g., marketing approval). fLast study participant first dose. gDatabase lock June 2021